Question: PRACTICE EXAMPLE A: Identify, whether each of the following is an oxidation-reduction reaction (a) (NH4)2SO4(aq)+Ba(NO3)(aq)BaSO4(s)+2NH4NO3(aq)= (b) 2Pb2(NO3)2(s)2PbO(s)+4NO2(g)+O2(g) PRACTICE EXAMPLE B: Identify the species that is
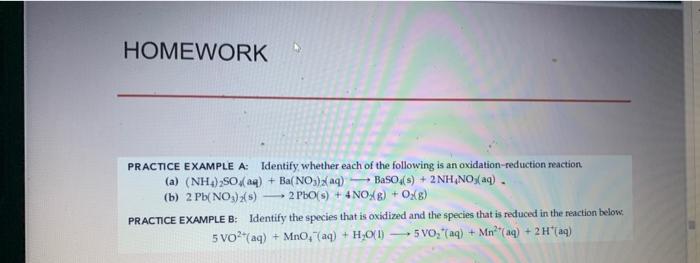
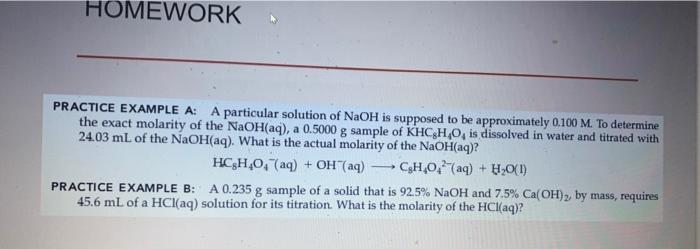
PRACTICE EXAMPLE A: Identify, whether each of the following is an oxidation-reduction reaction (a) (NH4)2SO4(aq)+Ba(NO3)(aq)BaSO4(s)+2NH4NO3(aq)= (b) 2Pb2(NO3)2(s)2PbO(s)+4NO2(g)+O2(g) PRACTICE EXAMPLE B: Identify the species that is oxidized and the species that is reduced in the reaction below: 5VO2+(aq)+MnO4(aq)+H2O(l)5VO2+(aq)+Mn2+(aq)+2H+(aq) PRACTICE EXAMPLE A: A particular solution of NaOH is supposed to be approximately 0.100M. To determine the exact molarity of the NaOH(aq), a 0.5000g sample of KHC8H4O4 is dissolved in water and titrated with 24.03mL of the NaOH(aq). What is the actual molarity of the NaOH(aq) ? HC8H4O4(aq)+OH(aq)C8H4O42(aq)+H2O(l) PRACTICE EXAMPLE B: A 0.235g sample of a solid that is 92.5%NaOH and 7.5%Ca(OH)2, by mass, requires 45.6mL of a HCl(aq) solution for its titration. What is the molarity of the HCl(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


