Question: Predict and calculate the effect of concentration changes on an equilibrium system. Some (CH3)2CHOH is allowed to dissociate into (CH3)2CO and H2 at 452K. At
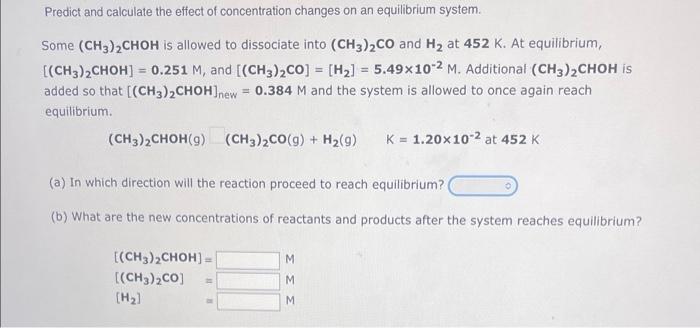
Predict and calculate the effect of concentration changes on an equilibrium system. Some (CH3)2CHOH is allowed to dissociate into (CH3)2CO and H2 at 452K. At equilibrium, [(CH3)2CHOH]=0.251M, and [(CH3)2CO]=[H2]=5.49102M. Additional (CH3)2CHOH is added so that [(CH3)2CHOH]new=0.384M and the system is allowed to once again reach equilibrium. (CH3)2CHOH(g)(CH3)2CO(g)+H2(g)K=1.20102at452K (a) In which direction will the reaction proceed to reach equilibrium? (b) What are the new concentrations of reactants and products after the system reaches equilibrium? [(CH3)2CHOH]=[(CH3)2CO]=[H2]MMM
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


