Question: Predict the equilibrium constant for the first reaction shown here given the equilibrium constants for the second and third reactions: CO2(g)+3H2(g)CH3OH(g)+H2O(g),K1=?CO(g)+H2O(g)CO2(g)H2(g),K2=1.2105CO(g)+2H2(g)CH3OH(g),K3=1.3107 Express the equilibrium constant

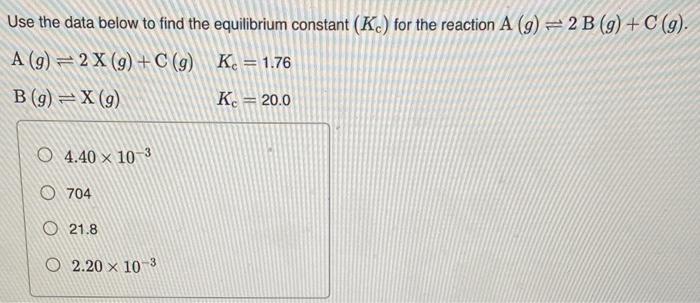
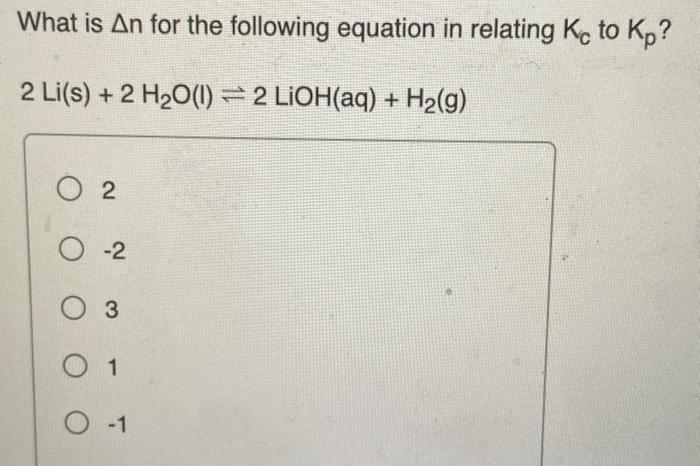

Predict the equilibrium constant for the first reaction shown here given the equilibrium constants for the second and third reactions: CO2(g)+3H2(g)CH3OH(g)+H2O(g),K1=?CO(g)+H2O(g)CO2(g)H2(g),K2=1.2105CO(g)+2H2(g)CH3OH(g),K3=1.3107 Express the equilibrium constant to two significant figures. Use the data below to find the equilibrium constant (Kc) for the reaction A(g)2B(g)+C(g). A(g)2X(g)+C(g)B(g)X(g)Kc=1.76Kc=20.0 4.40103 704 21.8 2.20103 What is n for the following equation in relating Kc to Kp ? 2Li(s)+2H2O(l)2LiOH(aq)+H2(g) 2 2 3 1 1 The reaction below has a Kp value of 41 . What is the value of Kc for this reaction at 400K ? N2(g)+3H2(g)2NH3(g) 2.3105 4.4104 41 26 3.8102
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


