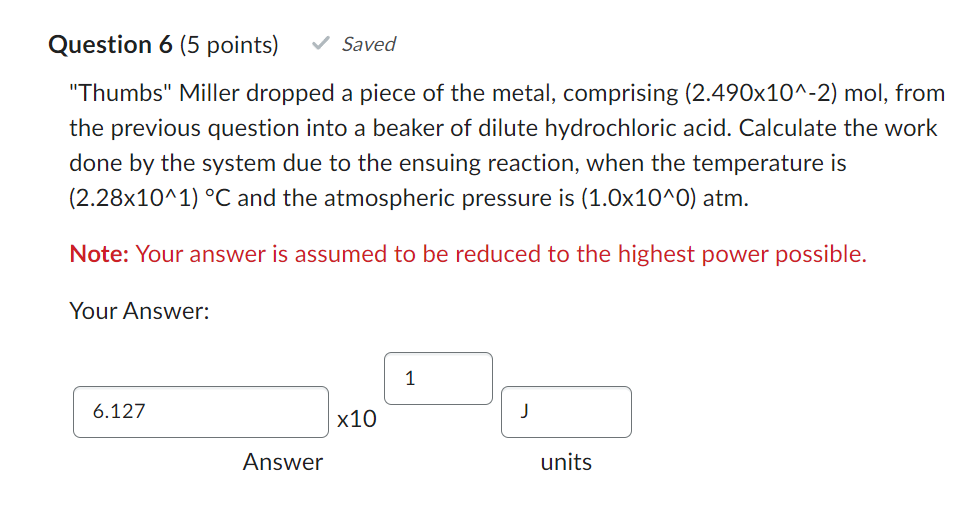
Question: PREVIOUS EQUATION: 2HCl + Mg MgCl2 + H2 Please help!! Thumbs Miller dropped a piece of the metal, comprising (2.490102)mol, from the previous question into
PREVIOUS EQUATION:
2HCl + Mg MgCl2 + H2
Please help!!
"Thumbs" Miller dropped a piece of the metal, comprising (2.490102)mol, from the previous question into a beaker of dilute hydrochloric acid. Calculate the work done by the system due to the ensuing reaction, when the temperature is (2.28101)C and the atmospheric pressure is (1.0100)atm. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: 10 Answer units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


