Question: *PREVIOUS EQUATION POSTED ABOVE* Pls answer to #6 Write a balanced equation for the reaction of hydrochloric acid, HCl(aq), with magnesium, a Group 2 metal,
*PREVIOUS EQUATION POSTED ABOVE* Pls answer to #6
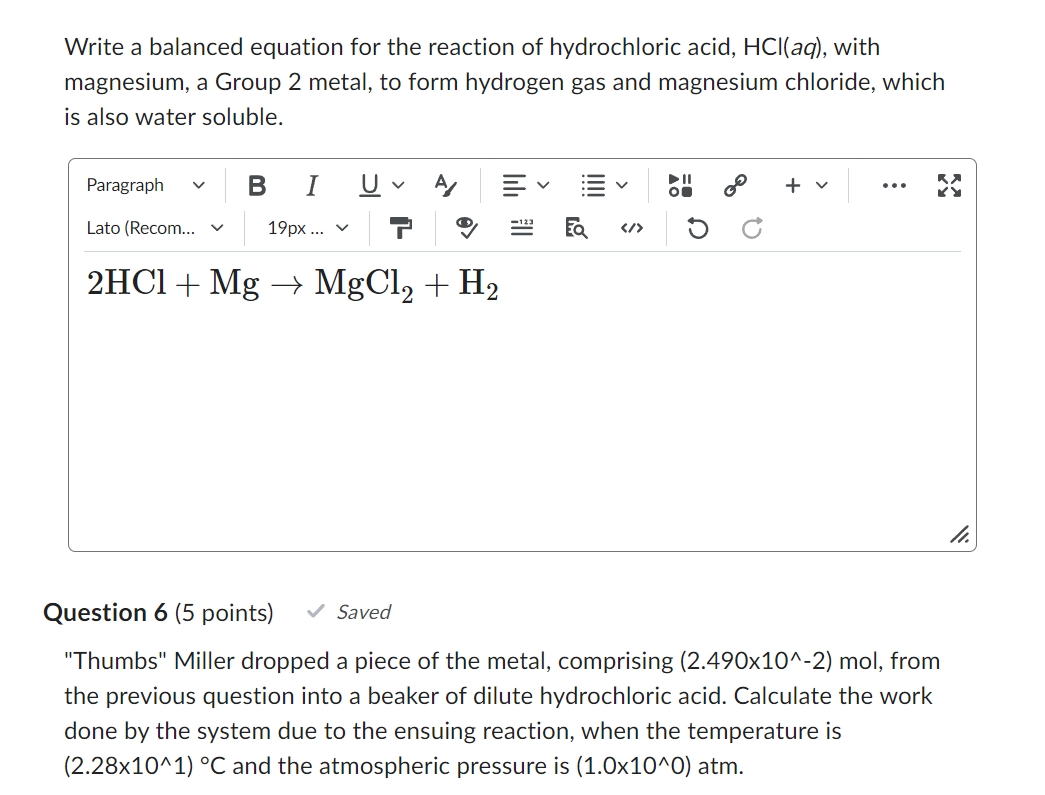
Write a balanced equation for the reaction of hydrochloric acid, HCl(aq), with magnesium, a Group 2 metal, to form hydrogen gas and magnesium chloride, which is also water soluble. Question 6 (5 points) Saved "Thumbs" Miller dropped a piece of the metal, comprising (2.490102)mol, from the previous question into a beaker of dilute hydrochloric acid. Calculate the work done by the system due to the ensuing reaction, when the temperature is (2.28101)C and the atmospheric pressure is (1.0100)atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


