Question: Previous person didn't help. I need the process by which you get the answer + the answers. I believe you have to use Raoult's Law
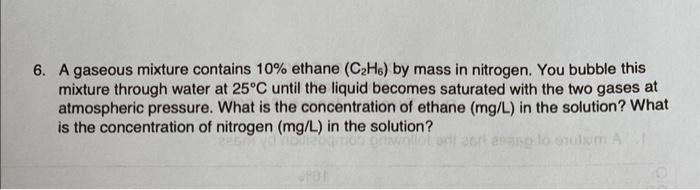
6. A gaseous mixture contains 10% ethane (C2H6) by mass in nitrogen. You bubble this mixture through water at 25C until the liquid becomes saturated with the two gases at atmospheric pressure. What is the concentration of ethane (mg/L) in the solution? What is the concentration of nitrogen (mg/L) in the solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


