Question: (previous question) . Fill the following reaction table which represents a reaction between HF and 0.02 mol of NaOH added to 500 mL of the
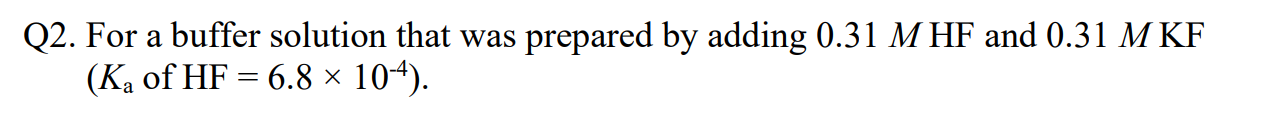
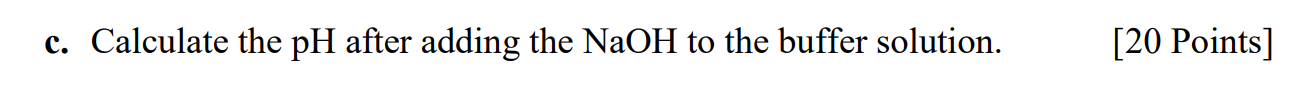
(previous question)
. Fill the following reaction table which represents a reaction between HF and 0.02 mol of NaOH added to 500 mL of the buffer solution.
Q2. For a buffer solution that was prepared by adding 0.31 M HF and 0.31 M KF (Ka of HF = 6.8 ~ 104). c. Calculate the pH after adding the NaOH to the buffer solution. [20 Points]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


