Question: Problem 1. (100 points) Renewable water electrolysis design Polymer exchange membrane (PEM) electrolyzers are based on a membrane electrode assembly that separates an O2 and
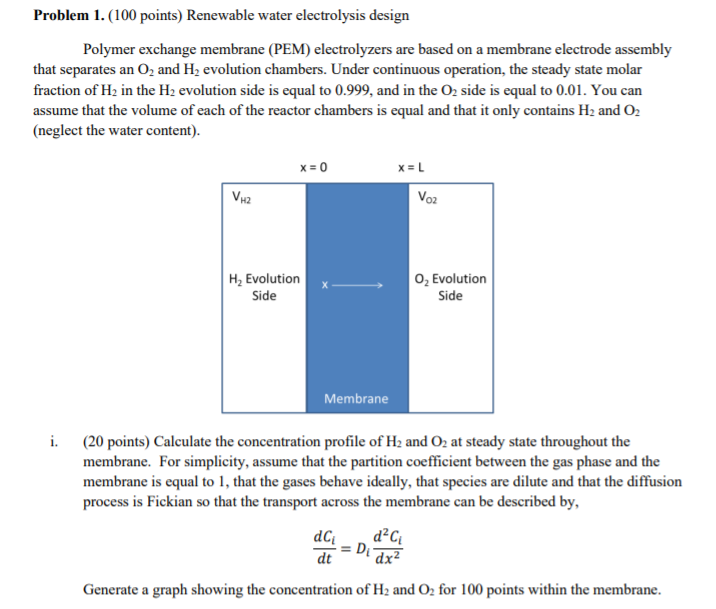
Problem 1. (100 points) Renewable water electrolysis design Polymer exchange membrane (PEM) electrolyzers are based on a membrane electrode assembly that separates an O2 and H, evolution chambers. Under continuous operation, the steady state molar fraction of H2 in the H2 evolution side is equal to 0.999, and in the 02 side is equal to 0.01. You can assume that the volume of each of the reactor chambers is equal and that it only contains H2 and 02 (neglect the water content). x=0 x=L Voz VH2 H, Evolution Side O Evolution Side Membrane i. (20 points) Calculate the concentration profile of H2 and O2 at steady state throughout the membrane. For simplicity, assume that the partition coefficient between the gas phase and the membrane is equal to 1, that the gases behave ideally, that species are dilute and that the diffusion process is Fickian so that the transport across the membrane can be described by, dC d2C D dt 'dx Generate a graph showing the concentration of H2 and O2 for 100 points within the membrane
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


