Question: Problem 1 (15 points) Consider the endothermic, liquid-phase, elementary dissociation reaction of A to form 2 molecules of B A - 2B (Reversible reaction) The
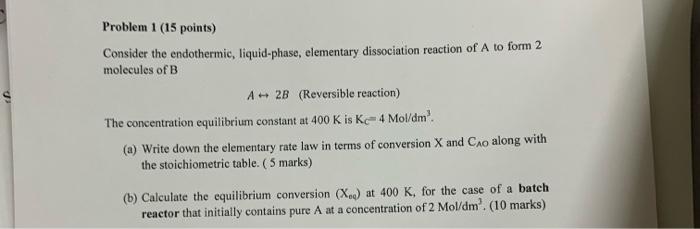
Problem 1 (15 points) Consider the endothermic, liquid-phase, elementary dissociation reaction of A to form 2 molecules of B A - 2B (Reversible reaction) The concentration equilibrium constant at 400 K is K-4 Mol/dm? (a) Write down the elementary rate law in terms of conversion X and Cao along with the stoichiometric table. (5 marks) (b) Calculate the equilibrium conversion (X.) at 400 K, for the case of a batch reactor that initially contains pure A at a concentration of 2 Mol/dm'. (10 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


