Question: Problem 1 (20 points) a. What is the mass in grams of a molecule of uranyl sulfate, UOSO4? What is this mass in amu?
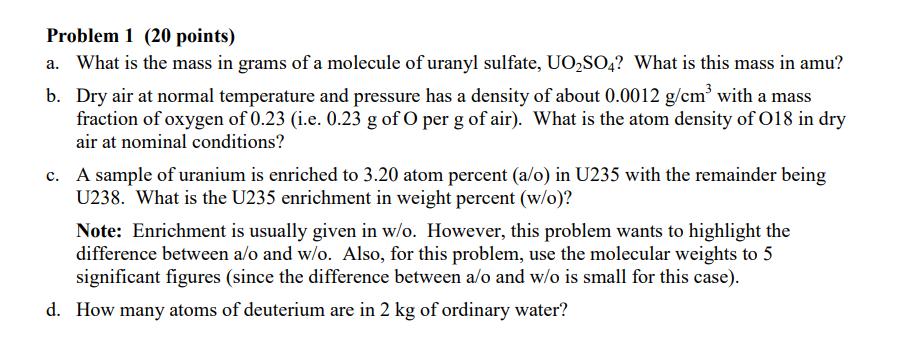
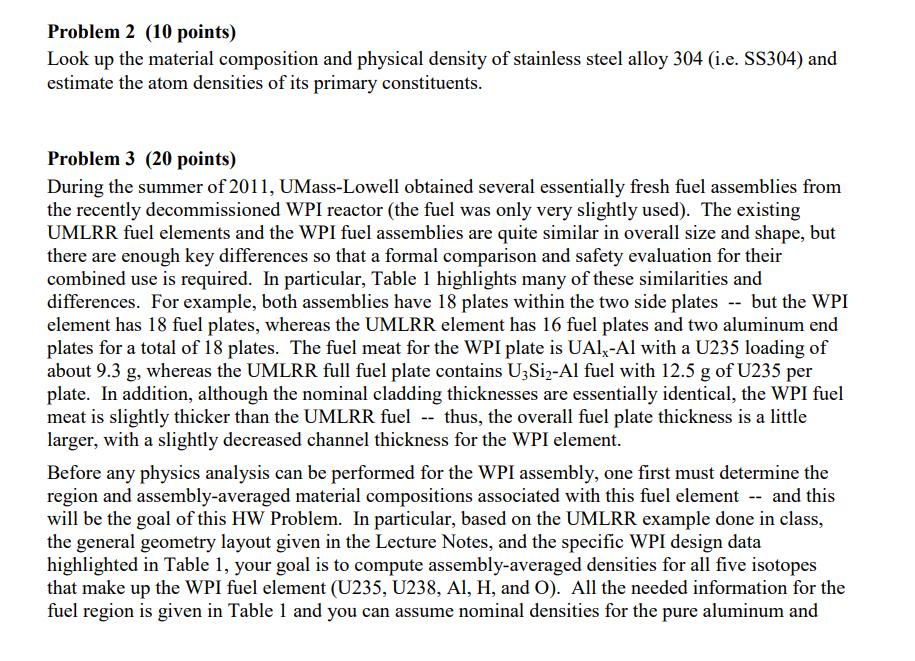
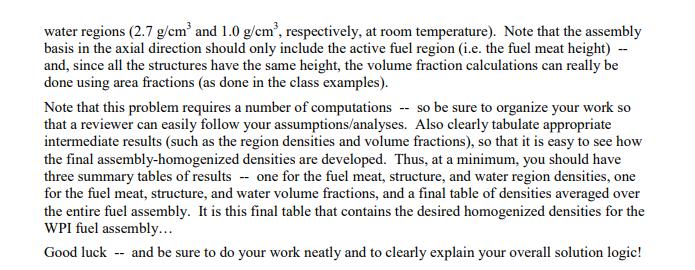
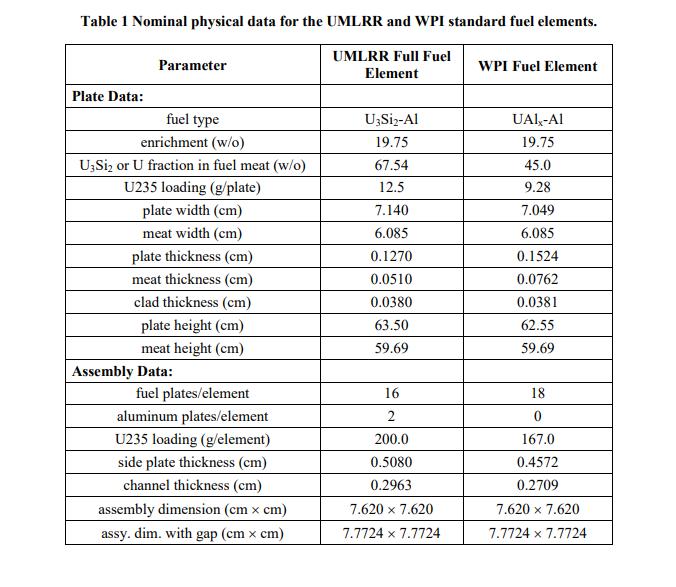
Problem 1 (20 points) a. What is the mass in grams of a molecule of uranyl sulfate, UOSO4? What is this mass in amu? b. Dry air at normal temperature and pressure has a density of about 0.0012 g/cm with a mass fraction of oxygen of 0.23 (i.e. 0.23 g of O per g of air). What is the atom density of 018 in dry air at nominal conditions? c. A sample of uranium is enriched to 3.20 atom percent (a/o) in U235 with the remainder being U238. What is the U235 enrichment in weight percent (w/o)? Note: Enrichment is usually given in w/o. However, this problem wants to highlight the difference between a/o and w/o. Also, for this problem, use the molecular weights to 5 significant figures (since the difference between a/o and w/o is small for this case). d. How many atoms of deuterium are in 2 kg of ordinary water? Problem 2 (10 points) Look up the material composition and physical density of stainless steel alloy 304 (i.e. SS304) and estimate the atom densities of its primary constituents. Problem 3 (20 points) During the summer of 2011, UMass-Lowell obtained several essentially fresh fuel assemblies from the recently decommissioned WPI reactor (the fuel was only very slightly used). The existing UMLRR fuel elements and the WPI fuel assemblies are quite similar in overall size and shape, but there are enough key differences so that a formal comparison and safety evaluation for their combined use is required. In particular, Table 1 highlights many of these similarities and differences. For example, both assemblies have 18 plates within the two side plates -- but the WPI element has 18 fuel plates, whereas the UMLRR element has 16 fuel plates and two aluminum end plates for a total of 18 plates. The fuel meat for the WPI plate is UAI,-Al with a U235 loading of about 9.3 g, whereas the UMLRR full fuel plate contains U3Si2-Al fuel with 12.5 g of U235 per plate. In addition, although the nominal cladding thicknesses are essentially identical, the WPI fuel meat is slightly thicker than the UMLRR fuel -- thus, the overall fuel plate thickness is a little larger, with a slightly decreased channel thickness for the WPI element. Before any physics analysis can be performed for the WPI assembly, one first must determine the region and assembly-averaged material compositions associated with this fuel element -- and this will be the goal of this HW Problem. In particular, based on the UMLRR example done in class, the general geometry layout given in the Lecture Notes, and the specific WPI design data highlighted in Table 1, your goal is to compute assembly-averaged densities for all five isotopes that make up the WPI fuel element (U235, U238, Al, H, and O). All the needed information for the fuel region is given in Table 1 and you can assume nominal densities for the pure aluminum and water regions (2.7 g/cm and 1.0 g/cm, respectively, at room temperature). Note that the assembly basis in the axial direction should only include the active fuel region (i.e. the fuel meat height) -- and, since all the structures have the same height, the volume fraction calculations can really be done using area fractions (as done in the class examples). Note that this problem requires a number of computations -- so be sure to organize your work so that a reviewer can easily follow your assumptions/analyses. Also clearly tabulate appropriate intermediate results (such as the region densities and volume fractions), so that it is easy to see how the final assembly-homogenized densities are developed. Thus, at a minimum, you should have three summary tables of results -- one for the fuel meat, structure, and water region densities, one for the fuel meat, structure, and water volume fractions, and a final table of densities averaged over the entire fuel assembly. It is this final table that contains the desired homogenized densities for the WPI fuel assembly... Good luck -- and be sure to do your work neatly and to clearly explain your overall solution logic! Table 1 Nominal physical data for the UMLRR and WPI standard fuel elements. UMLRR Full Fuel Element Plate Data: Parameter fuel type enrichment (w/o) U3Si or U fraction in fuel meat (w/o) U235 loading (g/plate) plate width (cm) meat width (cm) plate thickness (cm) meat thickness (cm) clad thickness (cm) plate height (cm) meat height (cm) Assembly Data: fuel plates/element aluminum plates/element U235 loading (g/element) side plate thickness (cm) channel thickness (cm) assembly dimension (cm x cm) assy. dim. with gap (cm x cm) U3Si-Al 19.75 67.54 12.5 7.140 6.085 0.1270 0.0510 0.0380 63.50 59.69 16 2 200.0 0.5080 0.2963 7.620 7.620 7.7724 x 7.7724 WPI Fuel Element UAIX-AI 19.75 45.0 9.28 7.049 6.085 0.1524 0.0762 0.0381 62.55 59.69 18 0 167.0 0.4572 0.2709 7.620 7.620 7.7724 x 7.7724
Step by Step Solution
3.40 Rating (166 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


