Question: Problem 1: (25 points) It is desired to prepare polyester with the number average molecular weight, M, 5000 g/mol by reacting of 1 mol butane-1,4
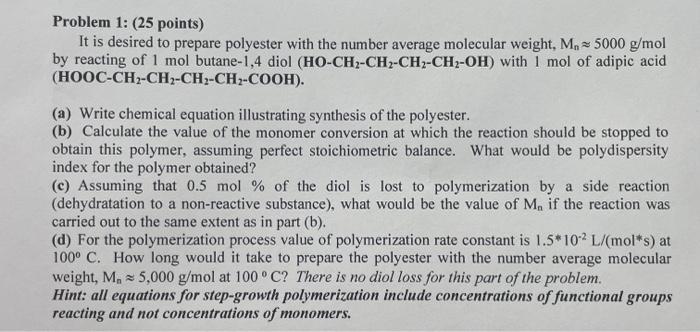
Problem 1: (25 points) It is desired to prepare polyester with the number average molecular weight, M, 5000 g/mol by reacting of 1 mol butane-1,4 diol (HO-CH2-CH2-CH2-CH2-OH) with 1 mol of adipic acid (HOOC-CH2-CH2-CH2-CH2-COOH). (a) Write chemical equation illustrating synthesis of the polyester. (b) Calculate the value of the monomer conversion at which the reaction should be stopped to obtain this polymer, assuming perfect stoichiometric balance. What would be polydispersity index for the polymer obtained? (c) Assuming that 0.5 mol % of the diol is lost to polymerization by a side reaction (dehydratation to a non-reactive substance), what would be the value of M. if the reaction was carried out to the same extent as in part (b). (d) For the polymerization process value of polymerization rate constant is 1.5*10-2 L/mol*s) at 100 C. How long would it take to prepare the polyester with the number average molecular weight, M. 25,000 g/mol at 100C? There is no diol loss for this part of the problem. Hint: all equations for step-growth polymerization include concentrations of functional groups reacting and not concentrations of monomers
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


