Question: Problem 1- 6 marks If you feed 10 grams N2 gas and 10 grams of H2 into a reactor as below: And considering that the
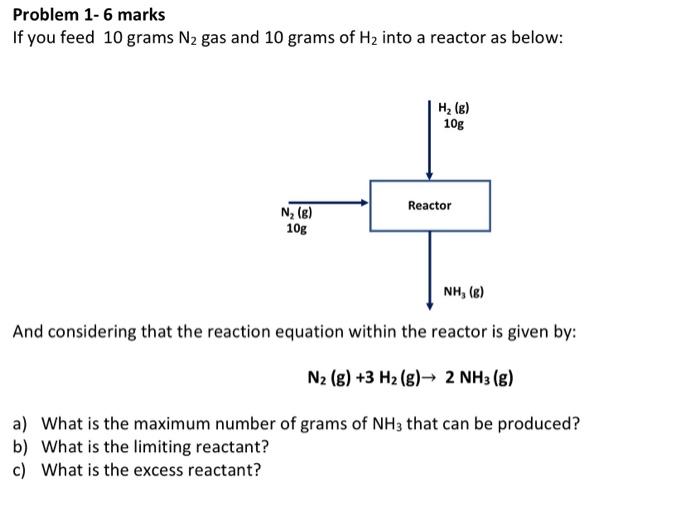
Problem 1- 6 marks If you feed 10 grams N2 gas and 10 grams of H2 into a reactor as below: And considering that the reaction equation within the reactor is given by: N2(g)+3H2(g)2NH3(g) a) What is the maximum number of grams of NH3 that can be produced? b) What is the limiting reactant? c) What is the excess reactant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


