Question: Problem 1 . 8 The van der Waals equation can be applied to describe the relationship between real gases over a range of temperature and
Problem
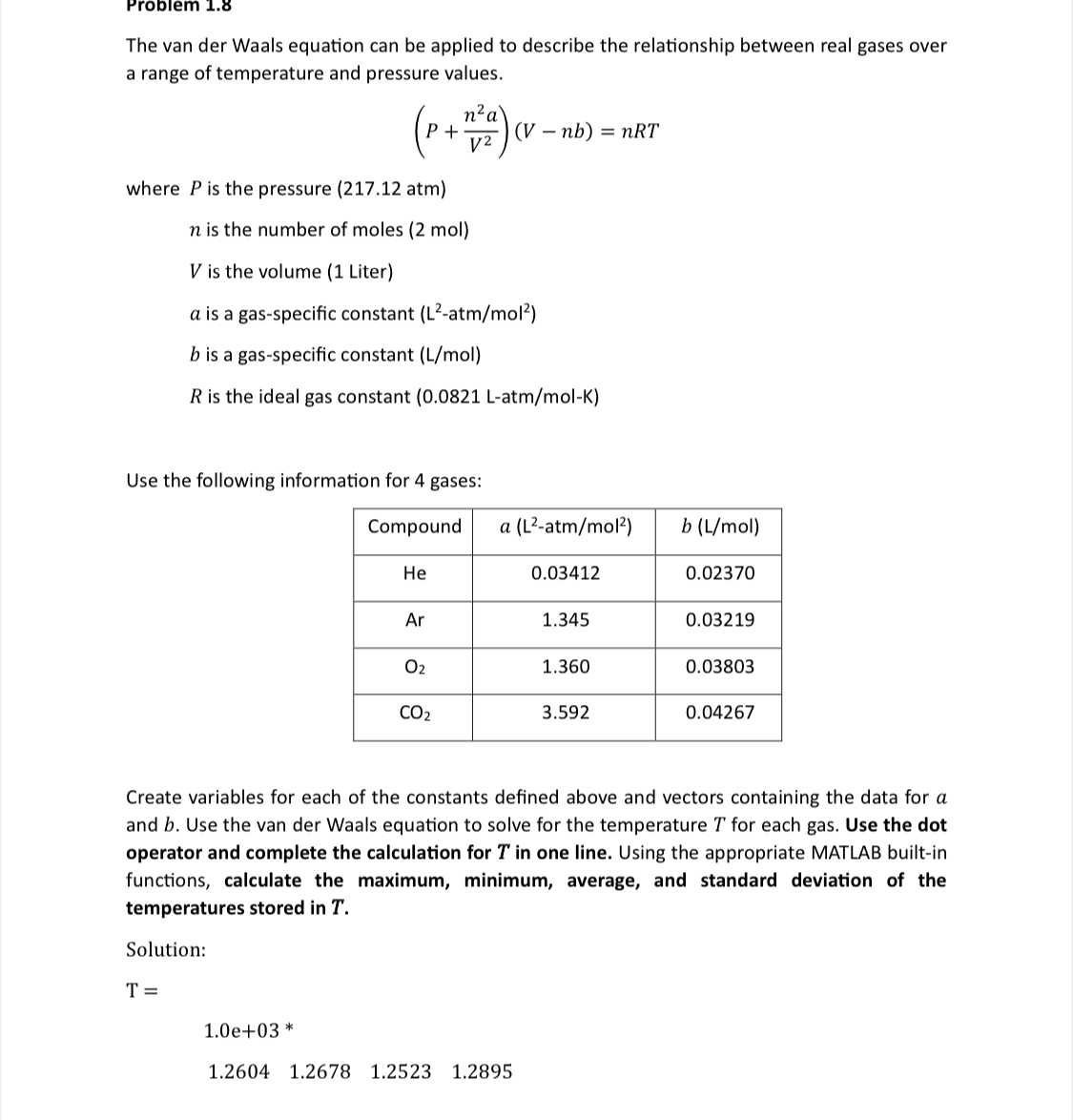
The van der Waals equation can be applied to describe the relationship between real gases over a range of temperature and pressure values.
where is the pressure atm
is the number of moles
is the volume Liter
is a gasspecific constant atmmol :
Solve it by matlab!
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


