Question: problem 8 MATLAB Problem (8): The van der Waals equation gives a relationship between the pressure p (atm), volume V (L), and temperature T (K)
problem 8 MATLAB
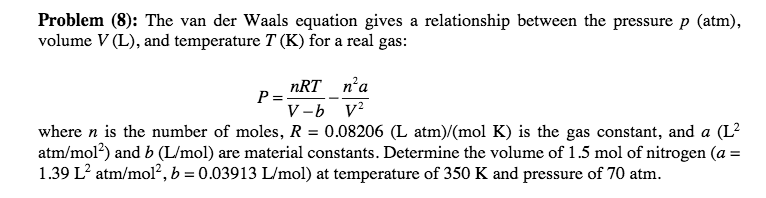
Problem (8): The van der Waals equation gives a relationship between the pressure p (atm), volume V (L), and temperature T (K) for a real gas: nRT n'a where n is the number of moles, R = 0.08206 (L atm)/(mol K) is the gas constant, and a (L2 atm/m012) and b (L/mol) are material constants. Determine the volume of 1 .5 mol of nitrogen (a- 1.39 L2 atm/mol, b = 0.03913 Lmol) at temperature of 350 K and pressure of 70 atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


