Question: Problem 1. Determine the enthalpy change Ah and he internal energy change Au of Air in kJ/kg, as it is heated from 283 to 298
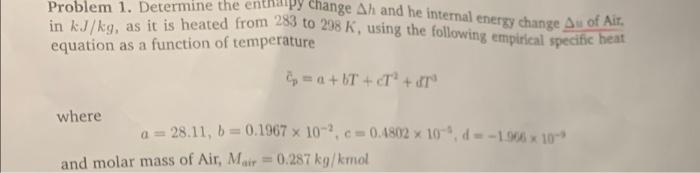
Problem 1. Determine the enthalpy change Ah and he internal energy change Au of Air in kJ/kg, as it is heated from 283 to 298 K, using the following empirical specific heat equation as a function of temperature =a+bT+T + dr where a=28.11, b=0.1967 x 102, c= 0.4802 x 10, d=-1.966 x 10-* and molar mass of Air, Mair= 0.287 kg/kmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


