Question: Problem # 1 i . A 2 m 3 tank contains 7 kg of steam ( H 2 O ) . At pressure of 5
Problem #
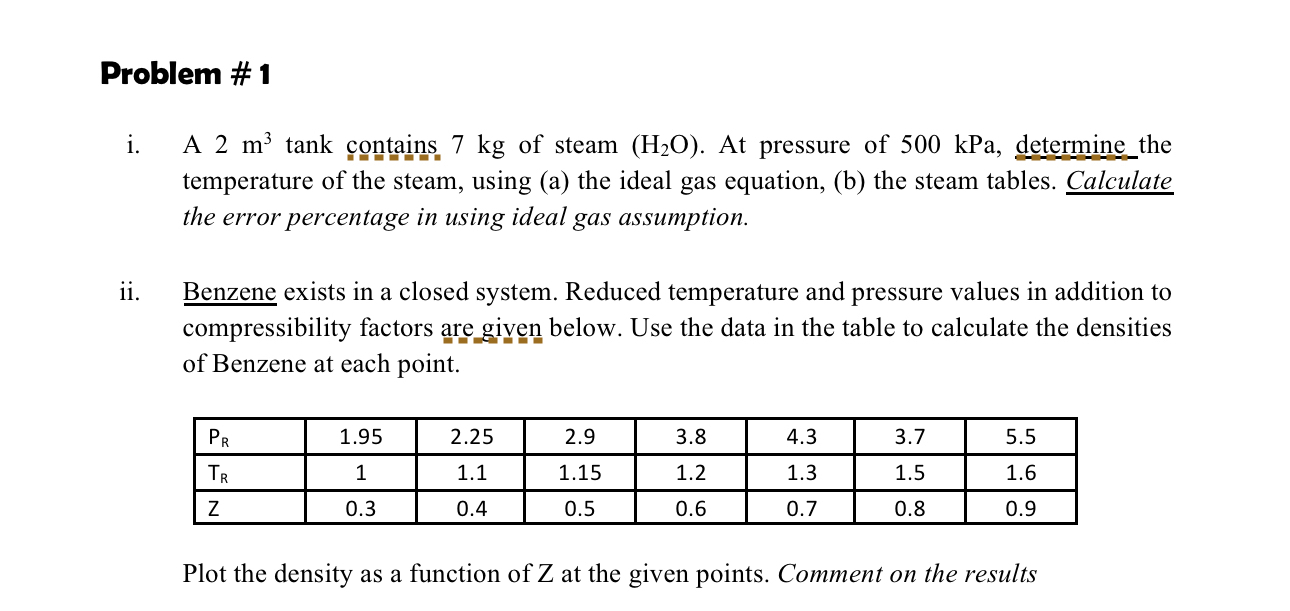
i A tank contains kg of steam At pressure of kPa determine the temperature of the steam, using a the ideal gas equation, b the steam tables. Calculate the error percentage in using ideal gas assumption.
ii Benzene exists in a closed system. Reduced temperature and pressure values in addition to compressibility factors are given below. Use the data in the table to calculate the densities of Benzene at each point.
tableZ
Plot the density as a function of Z at the given points. Comment on the results
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


