Question: Problem 1 Styrene is being polymerized at steady state in a 1 , 0 0 0 L continuous reactor that can be modeled as two
Problem
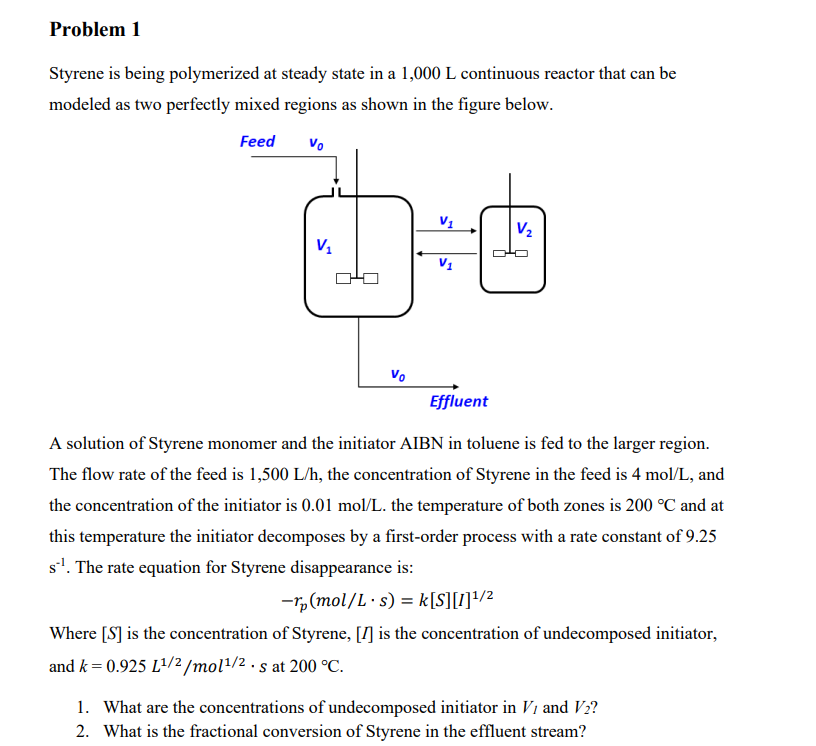
Styrene is being polymerized at steady state in a continuous reactor that can be
modeled as two perfectly mixed regions as shown in the figure below.
A solution of Styrene monomer and the initiator AIBN in toluene is fed to the larger region.
The flow rate of the feed is the concentration of Styrene in the feed is and
the concentration of the initiator is the temperature of both zones is and at
this temperature the initiator decomposes by a firstorder process with a rate constant of
The rate equation for Styrene disappearance is:
Where is the concentration of Styrene, I is the concentration of undecomposed initiator,
and at
What are the concentrations of undecomposed initiator in and
What is the fractional conversion of Styrene in the effluent stream?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


