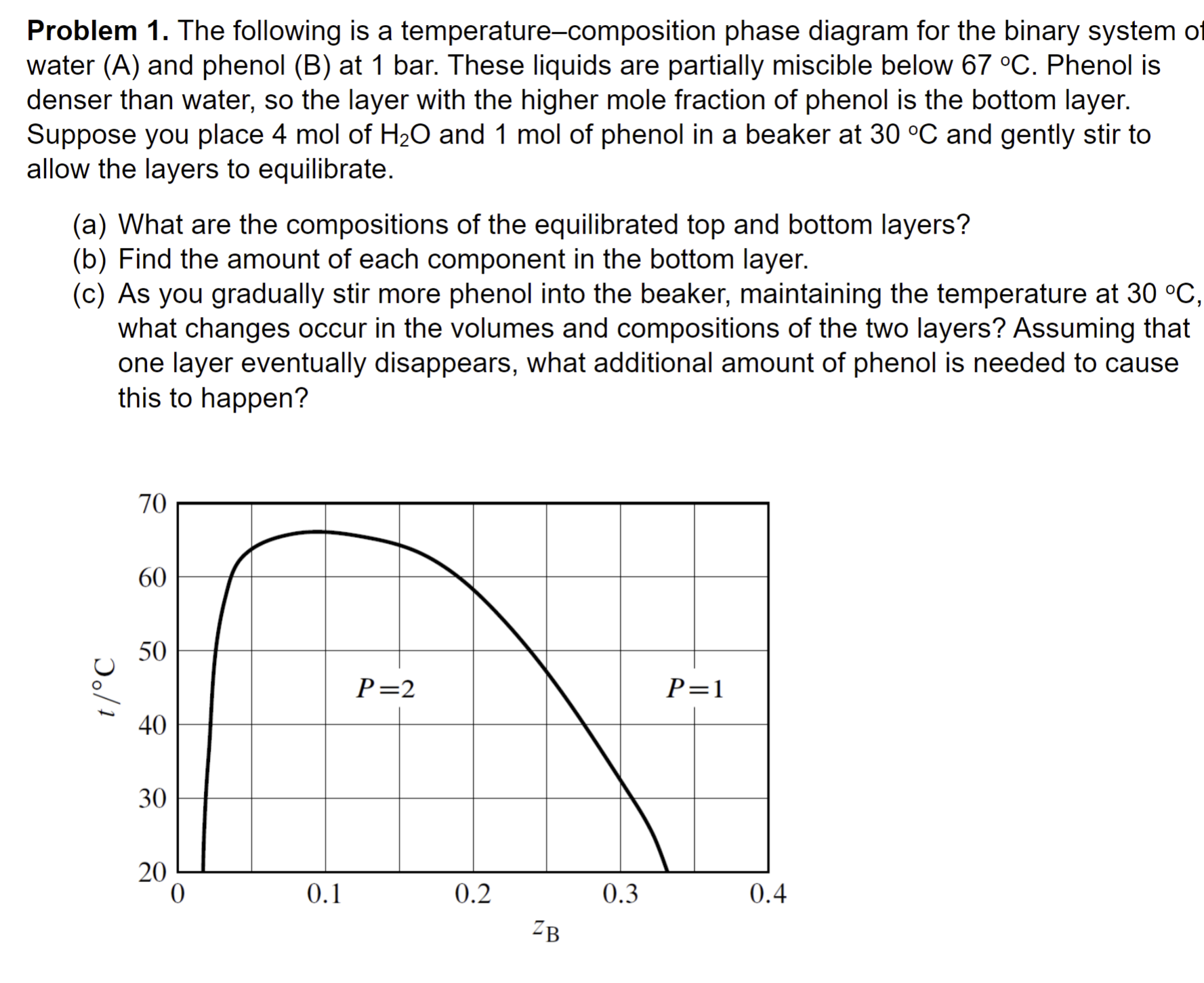
Question: Problem 1 . The following is a temperature - composition phase diagram for the binary system of water ( A ) and phenol ( B
Problem The following is a temperaturecomposition phase diagram for the binary system of water A and phenol B at bar. These liquids are partially miscible below Phenol is denser than water, so the layer with the higher mole fraction of phenol is the bottom layer. Suppose you place mol of and mol of phenol in a beaker at and gently stir to allow the layers to equilibrate.
a What are the compositions of the equilibrated top and bottom layers?
b Find the amount of each component in the bottom layer.
c As you gradually stir more phenol into the beaker, maintaining the temperature at what changes occur in the volumes and compositions of the two layers? Assuming that one layer eventually disappears, what additional amount of phenol is needed to cause this to happen?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


