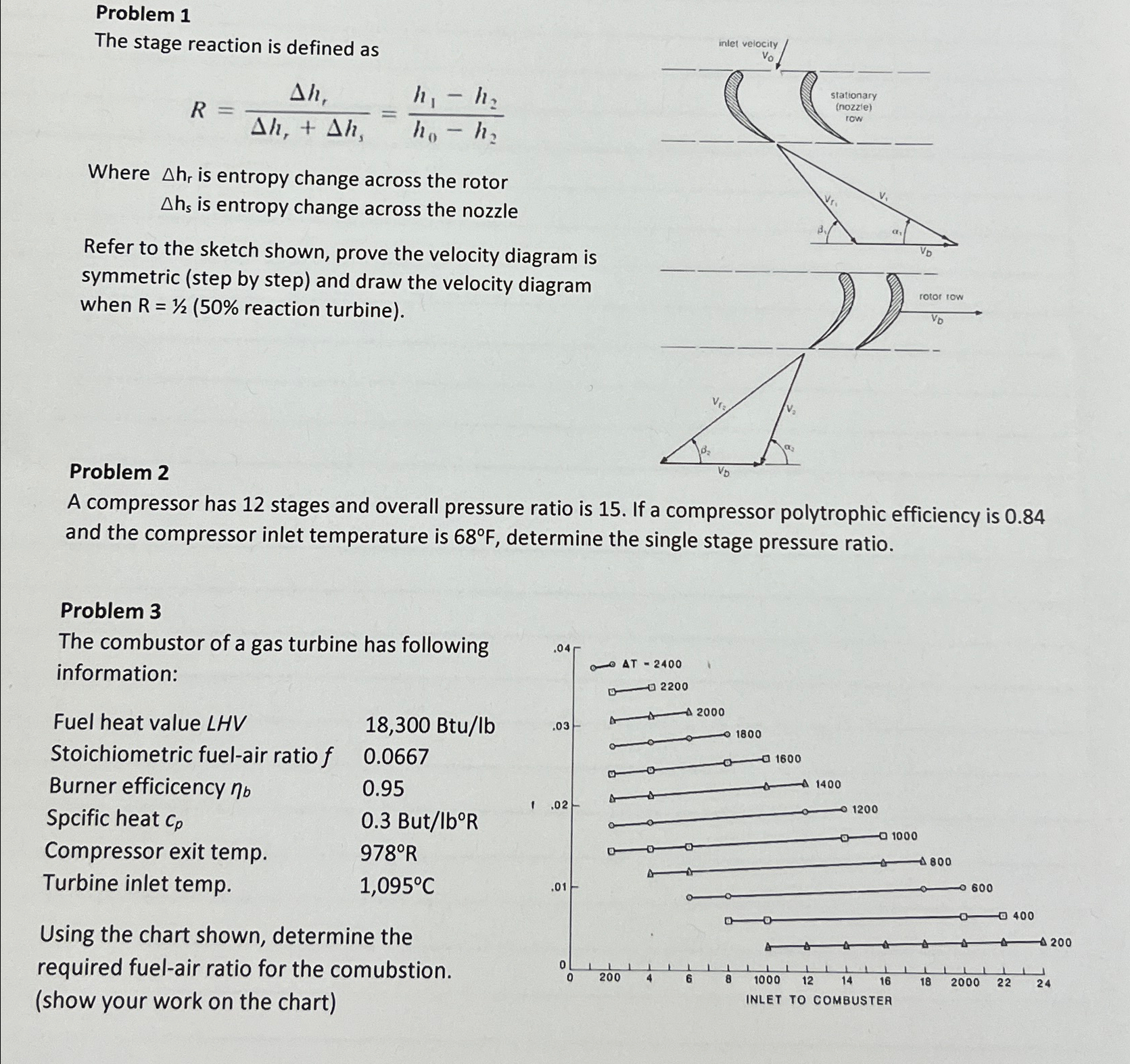
Question: Problem 1 The stage reaction is defined as R = h 1 h 1 + h s = h 1 - h 2 h 0
Problem
The stage reaction is defined as
Where is entropy change across the rotor
is entropy change across the nozzle
Refer to the sketch shown, prove the velocity diagram is symmetric step by step and draw the velocity diagram when reaction turbine
Problem
A compressor has stages and overall pressure ratio is If a compressor polytrophic efficiency is and the compressor inlet temperature is determine the single stage pressure ratio.
Problem
The combustor of a gas turbine has following information:
Fuel heat value LHV
Btulb
Stoichiometric fuelair ratio
Burner efficicency
Spcific heat
Butlbo
Compressor exit temp.
Turbine inlet temp.
Using the chart shown, determine the required fuelair ratio for the comubstion.
show your work on the chart
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


