Question: Problem 1. A second-order reaction in a gas phase: A+B>R (-r) kCACE k-5.1*10'exp(-6100/T) m/(kmol 1) takes place in an adiabatic PFR at 1000 KPa. The
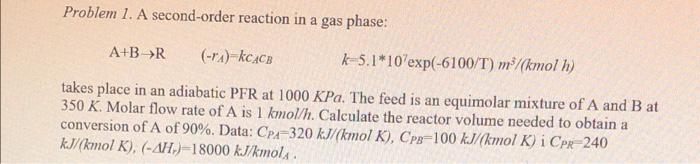
Problem 1. A second-order reaction in a gas phase: A+B>R (-r) kCACE k-5.1*10'exp(-6100/T) m/(kmol 1) takes place in an adiabatic PFR at 1000 KPa. The feed is an equimolar mixture of A and B at 350 K. Molar flow rate of A is 1 kmol/h. Calculate the reactor volume needed to obtain a conversion of A of 90%. Data: CP=320 kJ/(kmol K), Cp=100 kJ/(kmol K) i Cpr-240 kJ/kmol K), (-AH.)-18000 kJ/kmols
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


