Question: Problem 1. Using the distillation operation shown in Figure 1, calculate the following. a. Estimate the bubble point temperature of the distillate based on the
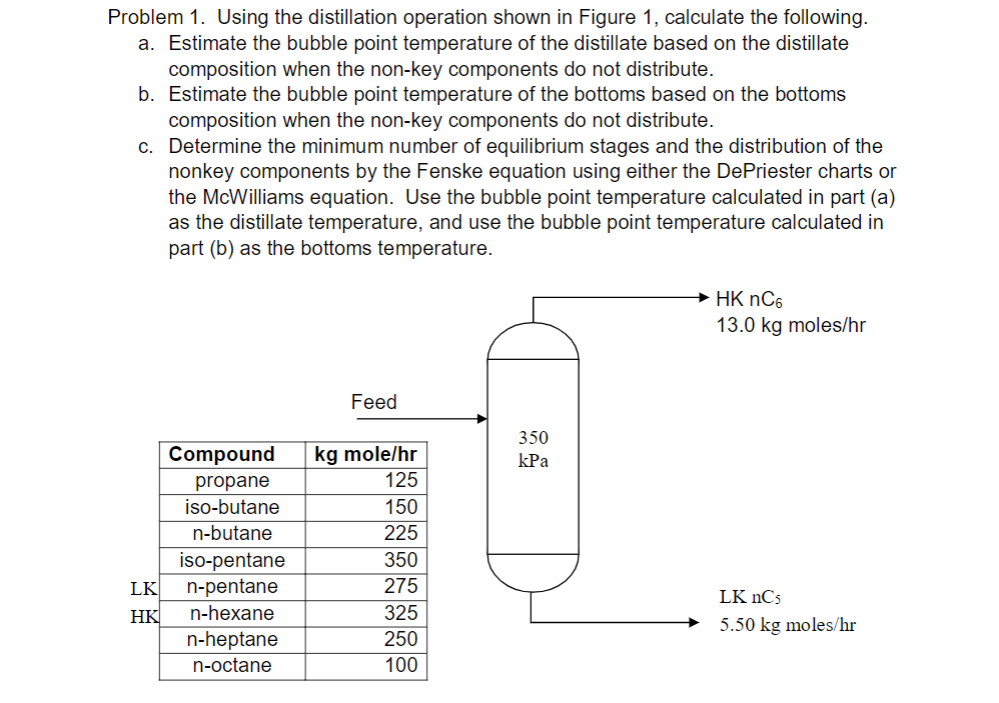
Problem 1. Using the distillation operation shown in Figure 1, calculate the following. a. Estimate the bubble point temperature of the distillate based on the distillate composition when the non-key components do not distribute. b. Estimate the bubble point temperature of the bottoms based on the bottoms composition when the non-key components do not distribute. c. Determine the minimum number of equilibrium stages and the distribution of the nonkey components by the Fenske equation using either the De Priester charts or the McWilliams equation. Use the bubble point temperature calculated in part (a) as the distillate temperature, and use the bubble point temperature calculated in part (b) as the bottoms temperature. HK nC6 13.0 kg moles/hr Feed 350 kPa Compound propane iso-butane n-butane iso-pentane LK n-pentane HK n-hexane n-heptane n-octane kg mole/hr 125 150 225 350 275 325 250 100 LK nCs 5.50 kg moles/hr
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


