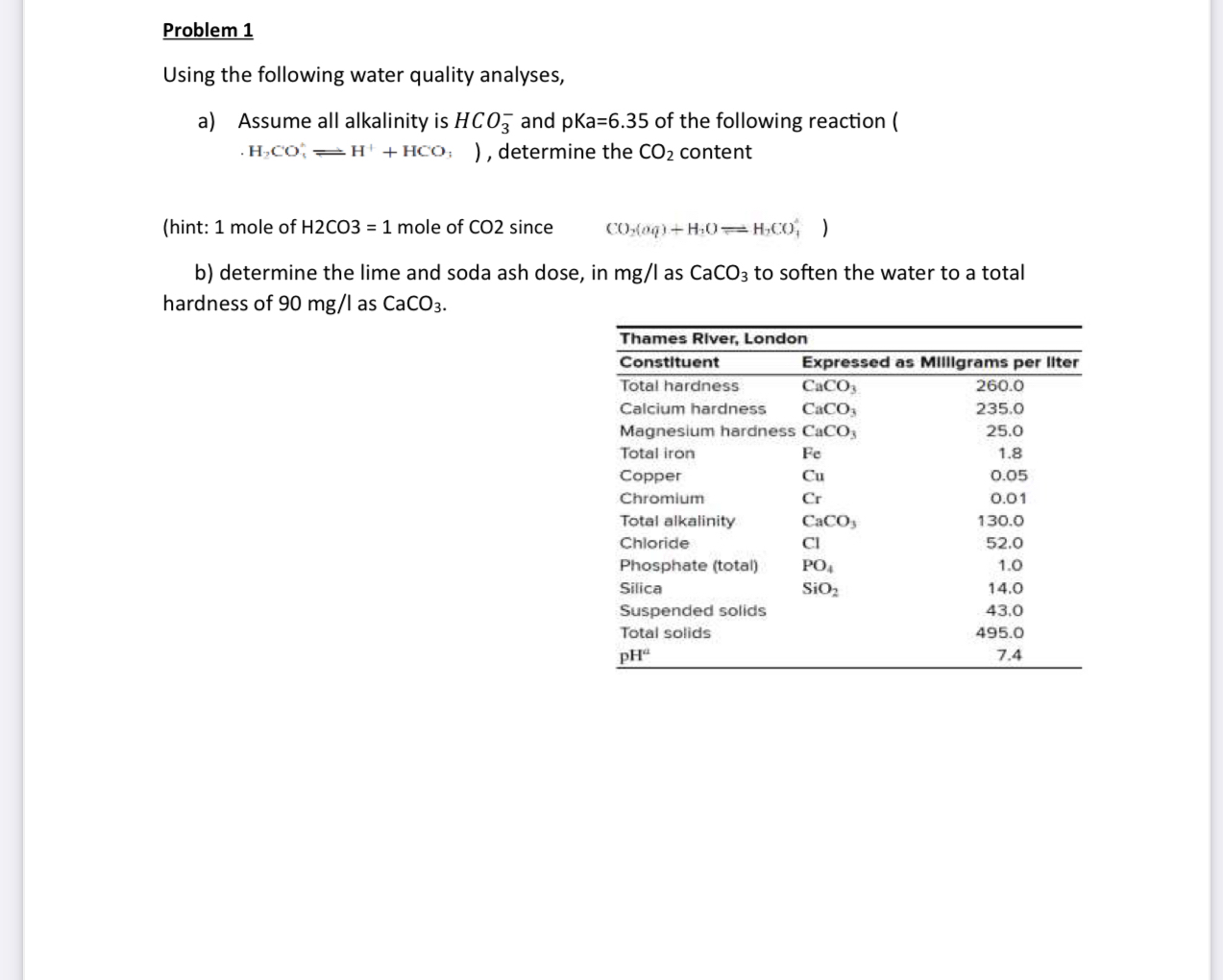
Question: Problem 1 Using the following water quality analyses, a ) Assume all alkalinity is H C O 3 - and pKa = 6 . 3
Problem
Using the following water quality analyses,
a Assume all alkalinity is and pKa of the following reaction determine the content
hint: mole of mole of since
:
b determine the lime and soda ash dose, in as to soften the water to a total hardness of as
tableThames RIver, LondontableConstituentTotal hardnessExpressed as Mililgrams per IIte!Calcium hardness,Magnesium hardiness,Total iron,CopperChromiumTotal alkalinity,ChloridePhosphate totalSilicaSuspended solids,,Total solids,,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


