Question: Problem 2 ( 1 0 points ) - Ideal gas closed system entropy analysis The figure below shows a thermally insulated box that is initially
Problem points Ideal gas closed system entropy analysis
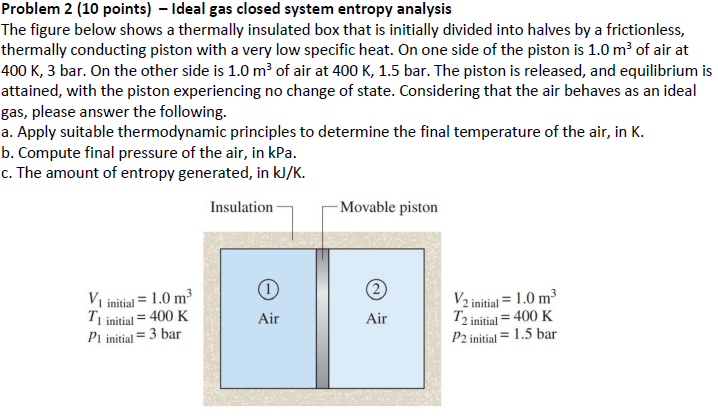
The figure below shows a thermally insulated box that is initially divided into halves by a frictionless, thermally conducting piston with a very low specific heat. On one side of the piston is mathrm~m of air at mathrm~Kmathrmbar On the other side is mathrm~m of air at mathrm~K bar. The piston is released, and equilibrium is attained, with the piston experiencing no change of state. Considering that the air behaves as an ideal gas, please answer the following.
a Apply suitable thermodynamic principles to determine the final temperature of the air, in K
b Compute final pressure of the air, in kPa
c The amount of entropy generated, in mathrmkJmathrmK
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


