Question: Problem 2 ( 1 4 points ) . It is required to design a fixed bed reactor in which the following reaction is carried out
Problem points It is required to design a fixed bed reactor in which the following reaction
is carried out in the gas phase: The reaction is first order with respect to A and
first order with respect to The engineer in charge of this task carries out a study in a fixed
bed reactor fed under a total molar flow rate of with a pressure of atm and
at a temperature of The reactor operates at constant temperature, with equimolar feed
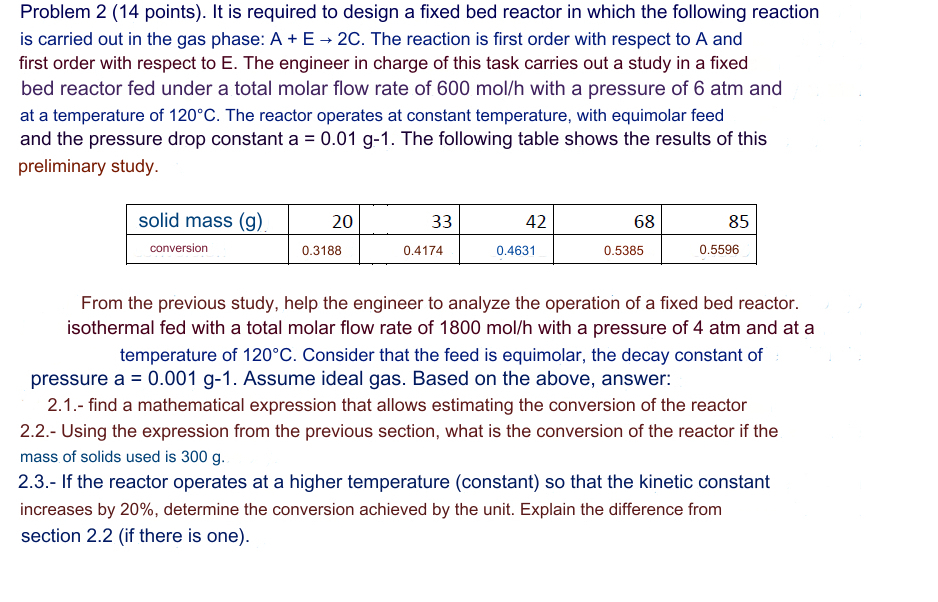
and the pressure drop constant The following table shows the results of this
preliminary study.
From the previous study, help the engineer to analyze the operation of a fixed bed reactor.
isothermal fed with a total molar flow rate of with a pressure of atm and at a
temperature of Consider that the feed is equimolar, the decay constant of
pressure Assume ideal gas. Based on the above, answer:
find a mathematical expression that allows estimating the conversion of the reactor
Using the expression from the previous section, what is the conversion of the reactor if the
mass of solids used is
If the reactor operates at a higher temperature constant so that the kinetic constant
increases by determine the conversion achieved by the unit. Explain the difference from
section if there is one
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


