There were 820 million pounds of phthalic anhydride produced in the United States in 1995. One of
Question:
There were 820 million pounds of phthalic anhydride produced in the United States in 1995. One of the end uses of phthalic anhydride is in the fiberglass of sailboat hulls. Phthalic anhydride can be produced by the partial oxidation of naphthalene in either a fixed or a fluidized catalytic bed. A flow sheet for the commercial process is shown below. Here, the reaction is carried out in fixed bed reactor with a vanadium pentoxide catalyst packed in 25 mm diameter tubes. A production rate of 31,000 tons per year would require 15,000 tubes.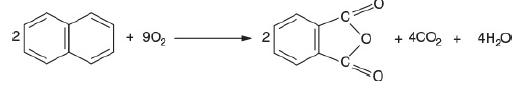
Two molecules of Naphthalene reacts with 9 molecules of oxygen (9O2) to give 2 molecules of phthalic anhydride (C6H4(CO)2O) plus 4 molecules of carbon-di-oxide
(4CO2) plus four molecules of water (4 H2O).
Assume the reaction is first order in oxygen and second order in naphthalene with kN = 0.01 dm6/mol2/s.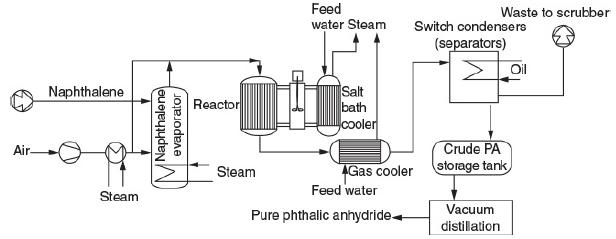
Naphthalene along with steam and air, is passed to the evaporator. Steam is passed to the evaporator and the naphthalene gets evaporated. Then it is moved to the reactor. The reactor is attached to a salt bath cooler. Water is fed to the salt bath and gas cooler. The reactor is attached with the gas cooler. Steam gets evaporated from the salt bath cooler and the gas cooler. From the gas cooler, the compound is passed to the switch condensers (separators), Oil is passed into the separators. Waste is released to the scrubber. From the separators, the mixture is sent to the crude PA storage tank, followed by vacuum distillation, and finally pure phthalic
anhydride is obtained. Adapted from Process Technology and Flowsheet Volume II. Reprints from Chemical Engineering, McGraw Hill Pub. Co., p. 111.
a. Set up a stoichiometric table for this reaction for an initial mixture of 3.5% naphthalene and 96.5% air (mol %), and use this table to develop the relations listed in parts (b) and (c). The initial/entering pressure and temperature are P0 = 10 atm and T0 = 500 K.
b. Express the following solely as a function of the conversion of naphthalene, X for a constant-volume isothermal batch reactor V = V0.
(1) The concentration of O2, CO2.
(2) The total pressure P.
(3) The rate of reaction, –rN.
(4) Then calculate the time to achieve 90% conversion.
c. Repeat (b) for an isothermal flow reactor to find CO2, volumetric flow rate and the reaction rate –rN as a function of conversion.
d. What CSTR and PFR volumes are necessary to achieve 98% conversion of naphthalene for an entering volumetric flow rate of 10 dm3/s.
Step by Step Answer:






