Question: Problem 2. (30 points) An irreversible, second order liquid phase reaction A + B occurs in a CSTR operated under the similar conditions as in
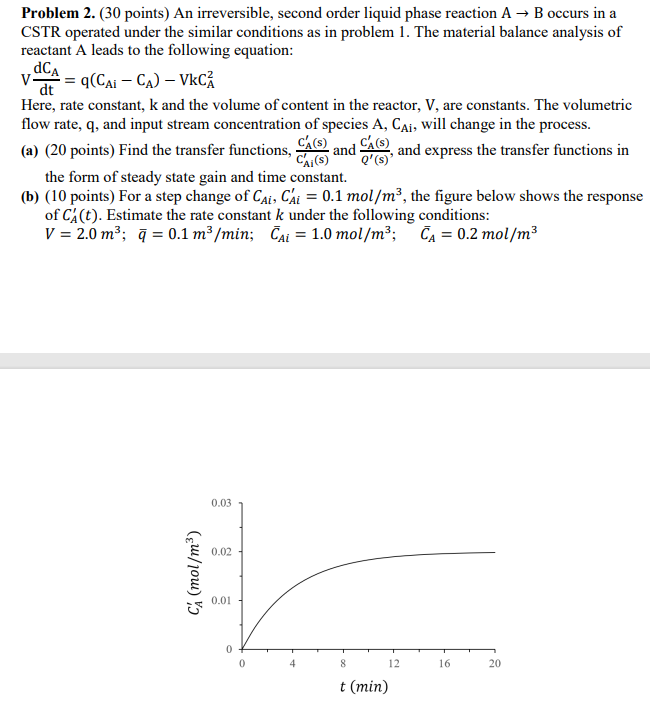
Problem 2. (30 points) An irreversible, second order liquid phase reaction A + B occurs in a CSTR operated under the similar conditions as in problem 1. The material balance analysis of reactant A leads to the following equation: V. CA vaca = qcCai Cw) VkC (- dt Here, rate constant, k and the volume of content in the reactor, V, are constants. The volumetric flow rate, q, and input stream concentration of species A, Cai, will change in the process. (a) (20 points) Find the transfer functions, ch(s) Cais) QUE , and express the transfer functions in the form of steady state gain and time constant. (b) (10 points) For a step change of Cai, Cai = 0.1 mol/m3, the figure below shows the response of Ca(t). Estimate the rate constant k under the following conditions: V = 2.0 m3; 7 = 0.1 m3/min; ai = 1.0 mol/m; CA = 0.2 mol/m3 and she 0.03 0.02 Ch (mol/m) 0.01 0 4 8 12 16 20 t (min)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


