Question: Problem 2. (30 points) Part 1 (10 pnts) How much heat is required to warm 0.50 L of water from 50.0 C to 100.0C? The
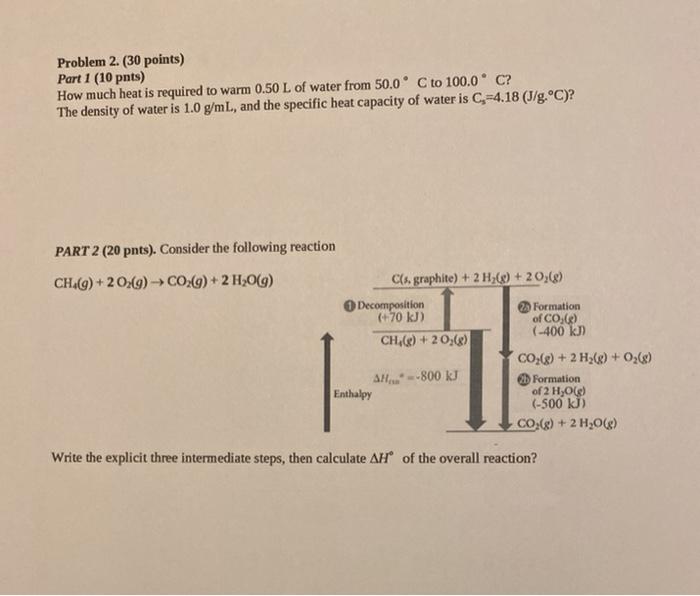
Problem 2. (30 points) Part 1 (10 pnts) How much heat is required to warm 0.50 L of water from 50.0 C to 100.0C? The density of water is 1.0 g/mL, and the specific heat capacity of water is C=4.18 (J/g.C)? PART 2 (20 pnts). Consider the following reaction CH() +20(9) CO2(g) + 2 H2O(9) C(s, graphite) + 2 H2(0)+20,() Decomposition 2 Formation (+70 kJ) of CO.) CH() +20,() (-400 kJ) CO2(8) + 2 H2(g) + O2(0) SH...--800 kJ Formation Enthalpy of 2 H,0) (-500 KJ) CO(8) + 2 H2008) Write the explicit three intermediate steps, then calculate AH of the overall reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


