Question: Problem 2 (3+3+2+2=10 points). Consider the cycle of forward first-order reactions: B a/ Write the rate equations controlling the evolution of the A, B, and
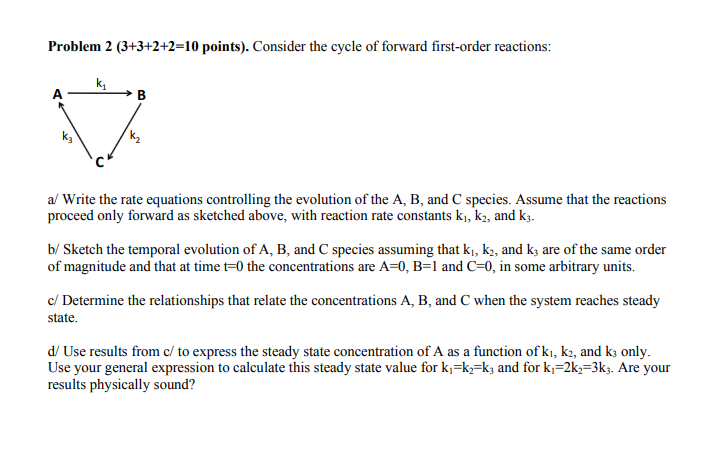
Problem 2 (3+3+2+2=10 points). Consider the cycle of forward first-order reactions: B a/ Write the rate equations controlling the evolution of the A, B, and species. Assume that the reactions proceed only forward as sketched above, with reaction rate constants k, k2, and kz. b/ Sketch the temporal evolution of A, B, and species assuming that k, k2, and k; are of the same order of magnitude and that at time t=0 the concentrations are A=0, B=1 and C=0, in some arbitrary units. c/ Determine the relationships that relate the concentrations A, B, and C when the system reaches steady state. d/ Use results from c/ to express the steady state concentration of A as a function of ki, k2, and k3 only. Use your general expression to calculate this steady state value for kj=k=kz and for k=2kz=3k3. Are your results physically sound
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


