Question: Problem 2 5 0 Marks Consider a mixture of methanol ( 1 ) , ethanol ( 2 ) and water ( 2 ) ternary system.
Problem
Marks
Consider a mixture of methanol ethanol and water ternary system. Assume ideal
behavior of the solution and Raoult's law can be applied.
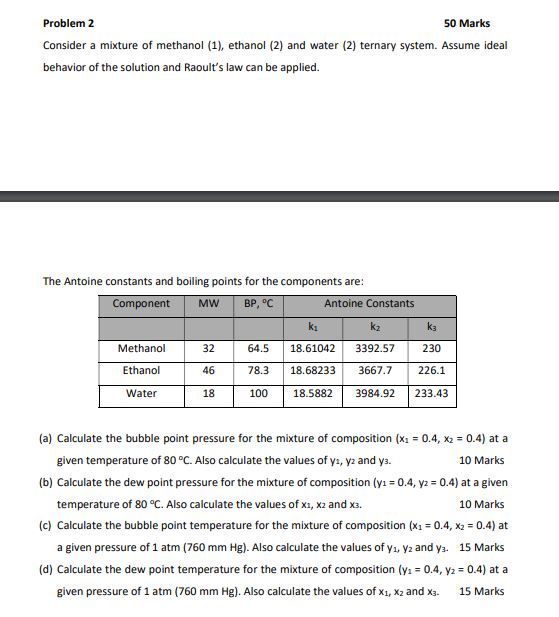
The Antoine constants and boiling points for the components are:
a Calculate the bubble point pressure for the mixture of composition at a
given temperature of Also calculate the values of and
Marks
b Calculate the dew point pressure for the mixture of composition : at a given
temperature of Also calculate the values of and
c Calculate the bubble point temperature for the mixture of composition at
a given pressure of atm Also calculate the values of and Marks
d Calculate the dew point temperature for the mixture of composition at a
given pressure of Also calculate the values of and Marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


