Question: Problem 2 (a) For each structure shown below draw all missing lone pairs and/or indicate the formal charge on any atom that requires one. Do
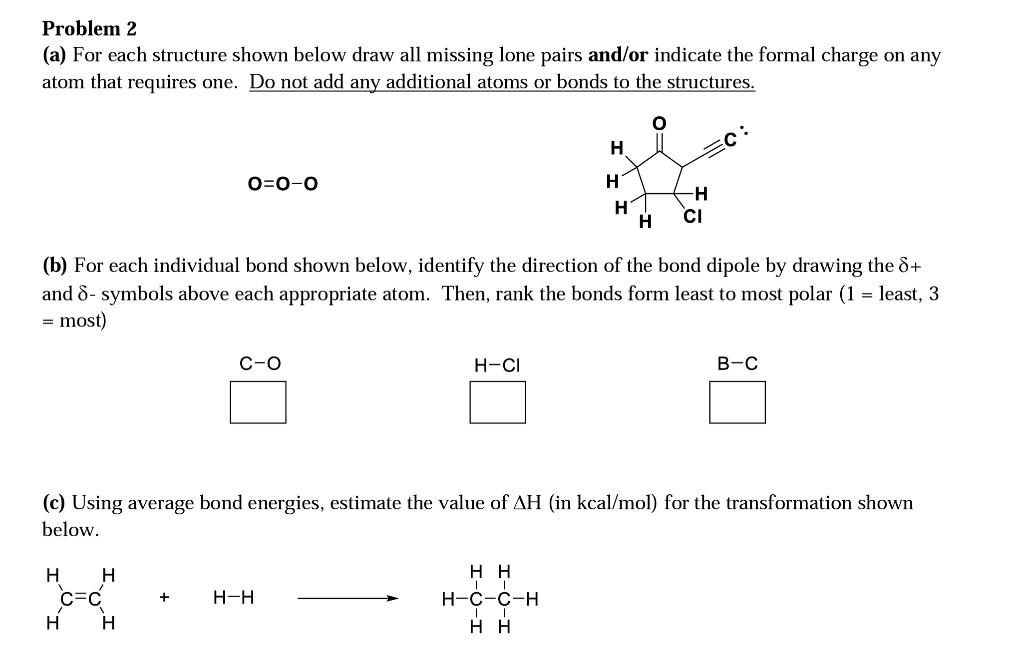
Problem 2 (a) For each structure shown below draw all missing lone pairs and/or indicate the formal charge on any atom that requires one. Do not add any additional atoms or bonds to the structures. 0 H O=0-0 H H -H CI H (b) For each individual bond shown below, identify the direction of the bond dipole by drawing the d+ and 8- symbols above each appropriate atom. Then, rank the bonds form least to most polar (1 = least, 3 = most) C-0 H-CI B-C (c) Using average bond energies, estimate the value of AH (in kcal/mol) for the transformation shown below. H . CEC H H + H-H HH H-C-C-H HH
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


