Question: Problem 2 : Consider a piston - cylinder assembly filled with 3 4 . 5 mol of a gas ( which has a critical pressure
Problem : Consider a pistoncylinder assembly filled with mol of a gas which has a
critical pressure of kPa and critical temperature of K with an initial temperature of
and an initial pressure of kPa state The gas is then compressed to a final
volume of state For parts i and ii use the ideal gas model. Use
K Find:
i The final temperature, in rounded to the nearest two decimal places, if
compression occurs at constant pressure call this state A
ii The final pressure, in kPa rounded to the nearest one decimal place, if compression
occurs at constant temperature call this State B
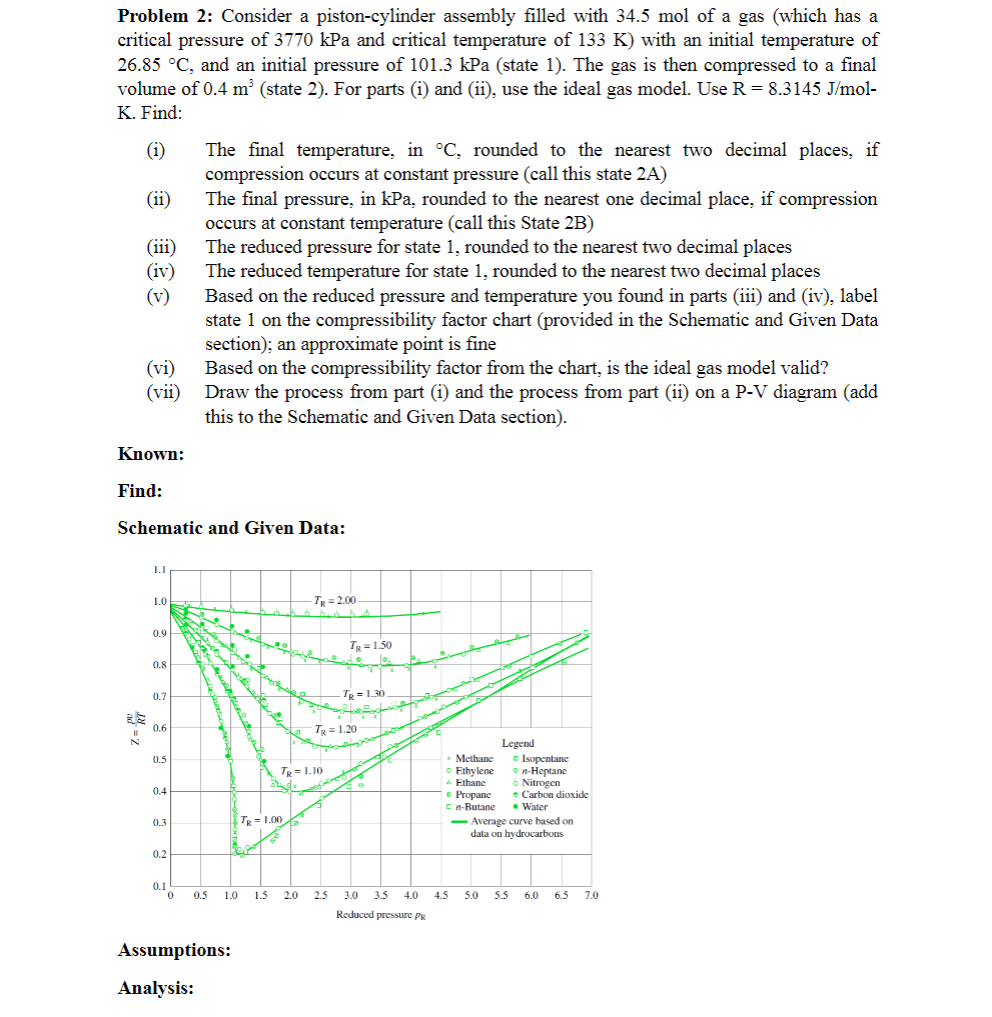
iii The reduced pressure for state rounded to the nearest two decimal places
iv The reduced temperature for state rounded to the nearest two decimal places
v Based on the reduced pressure and temperature you found in parts iii and iv label
state on the compressibility factor chart provided in the Schematic and Given Data
section; an approximate point is fine
vi Based on the compressibility factor from the chart, is the ideal gas model valid?
vii Draw the process from part i and the process from part ii on a PV diagram add
this to the Schematic and Given Data section
Known:
Find:
Schematic and Given Data:
Assumptions:
Analysis:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


