Question: Problem 2 In Homework 3 , you have worked with the aluminum - hydroxide coordination l o g C - p H diagram for coagulation
Problem In Homework you have worked with the aluminumhydroxide coordination diagram for coagulationflocculation water treatment, which is shown below. c pts The envelop curve is Total which is the sum of and
Because TotalAl varies with pH the concentration of most species can be presented as a function
of with other constants in the expression.
For example,
Please derive and using and the constants: and Show your process. dpts If citric acid see the structure below is added into the solution, do you expect the dissolved total Al to
be higher or lower? Why?
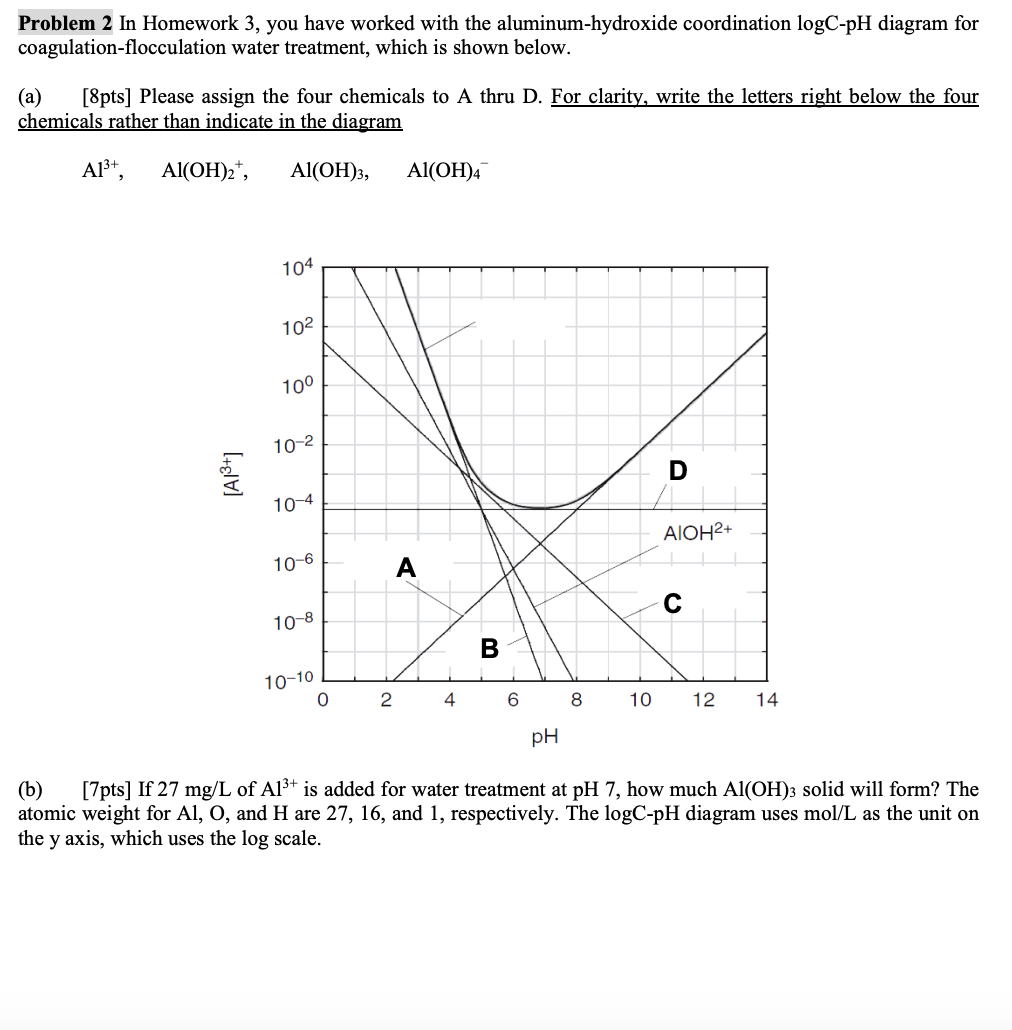
apts Please assign the four chemicals to A thru D For clarity, write the letters right below the four chemicals rather than indicate in the diagram
b If of is added for water treatment at pH how much solid will form? The atomic weight for and H are and respectively. The diagram uses as the unit on the axis, which uses the scale.
cpts The envelop curve is TotalAl which is the sum of Al AlOH
AlOH
AlOH and
AlOH
Because TotalAl varies with pH the concentration of most species can be presented as a function
of H
with other constants in the expression.
For example, AlOH
beta KspH
Kw
Please derive Al and AlOH
using H
and the constants: Kspbeta i and Kw Show your process
dpts If citric acid see the structure below is added into the solution, do you expect the dissolved total Al to
be higher or lower? Why?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


