Question: Problem 2. Product Separation and Recycle A and B are made to react in a reactor to produce Cand according to the reaction 2A +
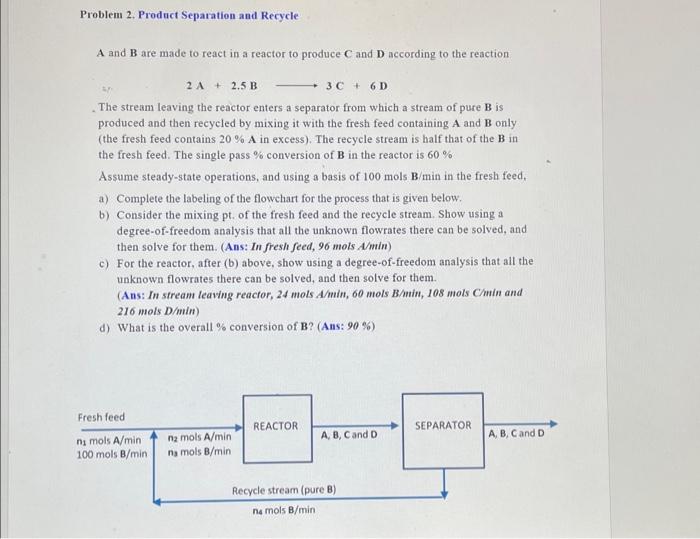
Problem 2. Product Separation and Recycle A and B are made to react in a reactor to produce Cand according to the reaction 2A + 2.5 B - 3C + 6D The stream leaving the reactor enters a separator from which a stream of pure Bis produced and then recycled by mixing it with the fresh feed containing A and B only (the fresh feed contains 20% A in excess). The recycle stream is half that of the Bin the fresh feed. The single pass % conversion of B in the reactor is 60 % Assume steady-state operations, and using a basis of 100 mols B/min in the fresh feed. a) Complete the labeling of the flowchart for the process that is given below. b) Consider the mixing pt. of the fresh feed and the recycle stream. Show using a degree of freedom analysis that all the unknown flowrates there can be solved, and then solve for them. (Ans: In fresh feed, 96 mois A/min) c) For the reactor, after (b) above, show using a degree-of-freedom analysis that all the unknown flowrates there can be solved, and then solve for them. (Ans: In stream leaving reactor, 24 mois A/min, 60 mols B/min, 108 mols C/min and 216 mols D/min) d) What is the overall % conversion of B? (Ans: 90 %) Fresh feed REACTOR SEPARATOR A, B, C and D A, B, C and D na mols A/min 100 mols B/min na mols A/min na mols B/min Recycle stream (pure B) ne mols B/min
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


