Question: Problem #2 Redo the in-class example assuming a dual site mechanism for the surface reaction. What do you specifically note that is different than what




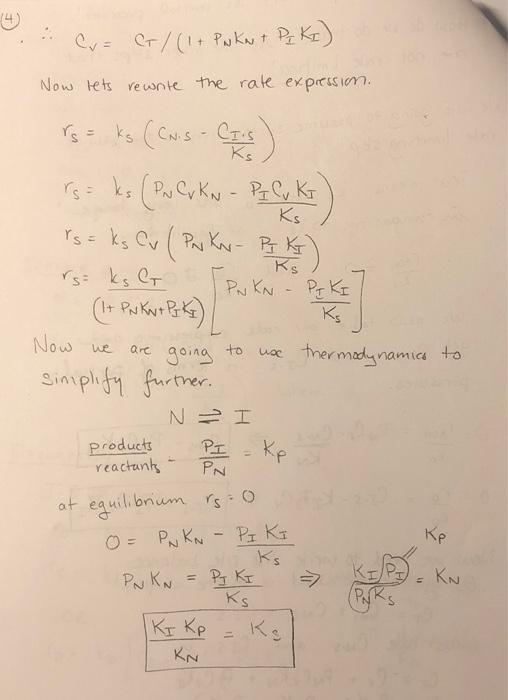
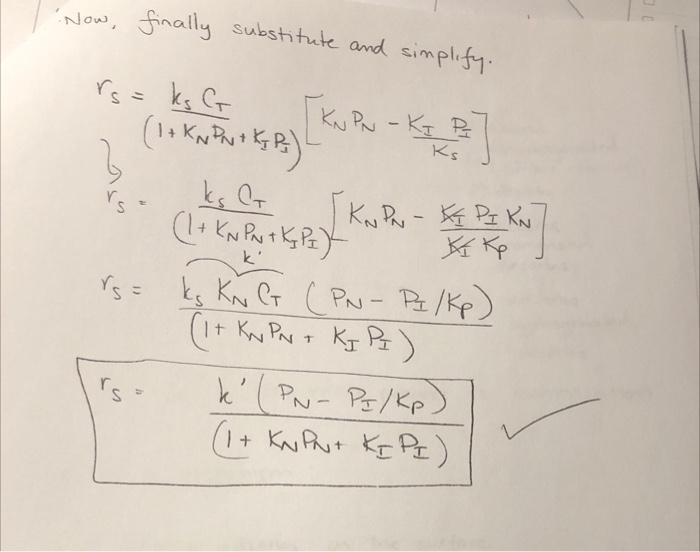
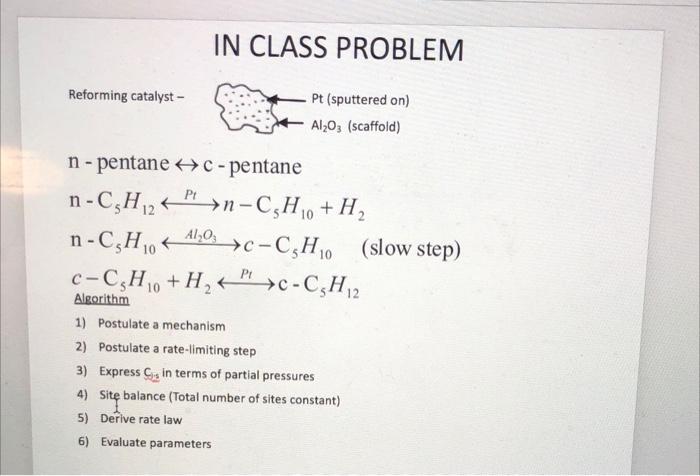
Problem #2 Redo the in-class example assuming a dual site mechanism for the surface reaction. What do you specifically note that is different than what we derived in class? IN CLASS EXAMPLE n-pentane no entene 11) adsorbs denydrogenated 2) (carte reaction 3) desorbs -> adsoros n-pentene c- pentene = isomerized c. pentene c- pentane desorbs ad corbs hydrogenated descubs The slow step in this entire process is i somenzaton Propose a mechanism : First: n-pentene adsorbs on the surface molecular adsorption N+S - N.S Second n-pentene is isomerized to form c-pentene N.SI.S (Single site, Surface Rxn for that slep. Third c-pentene desorbs from the surface I'S 2 I + S Next, write the rate expressions : ADSORPTION NS N.S VARN a Hachment- detachment TAON = KN PN Cv KCNS TAON where KN [PNG - CNS KN KN= KN forward 11 KN rever- SURFACE RON N. JOS Single Site! rs = ks CNS kis ks (CN.S - CIS where Kg Ks- kg Ks ks DESORPTION Jis I + S where ro = ko Ctis - k Cv P I roo ko [CI.s - Cu Pi ] roo ko [Cr.s - C v P K I ] ko ko OR and I KI to express Cis as partial How do we do that? Use the other steps that are not rate limiting! Press turco! Orc non is the slow We going to assume surface rate limiting step. That means KA and ko in comparison to ks at Equilibrian and DO very large YADN i o IN and ko We now take our rode expressions and get Cnis and Ctis in terms of partial Pressures. O=TAON = PNG- CNS KN KN CNS - PNC KN 0= ro IF CI.S - KPz Cv | CI.S = P Cv K | ko Now we need to write the sile balance e equilibrium what is on the surface! C = Cv + CNS + CI.S substitute Cris and CTS in. C = Cv + PNC v KN + B. Cuks CT = Cr(IT PNKN + PIKI) 4 Cu = C/ (1+ PN KN + PZ KI) w Now lets rewrite the rate expression. ro= ks (CNS - CTS Now we are = Ks rs= ks (PN C, KN - PE C y Kr Ks rs = ks Cv (PN K - PIK Ks rs= ks C PN KN - PF KI (1+ Pukno Pok Kg going to use thermodynamics to simplify further. NI products PI reactants PN at equilibrium rs: 0 0 = O = PNKN PNKN - PIK PN KN = PJ KI Ks (PNRs IKI Kp KN ke Ks KE - KN => Now, finally substitute and simplify. rs=ks G KNPN - KIP (1+ KN PN 1 KJP) Ks rs (1 + KNPN + Kg P =) k' } I KNPN - API KN & Kp rs= ks KNG (PN-Pq/kp) (it Kay Part Kg P2) k' (PN-Pz/kp) (1 + KNPut KIPI) 1 rs IN CLASS PROBLEM Reforming catalyst - Pt (sputtered on) Al2O3 (scaffold) - 10 A140; c-C,H, n - pentane HC-pentane n-C,H2+ PP >n-C,H,, + H, n-C,H0 + (slow step) C-C,H, +H,">C-C,H,2 Algorithm 1) Postulate a mechanism 2) Postulate a rate-limiting step 3) Express C in terms of partial pressures 4) Site balance (Total number of sites constant) 5) Derive rate law 6) Evaluate parameters
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


