Question: Problem 2. The schematic below shows a waste water treatment process associated with a clarifier. The goal of this waste water treatment process is to

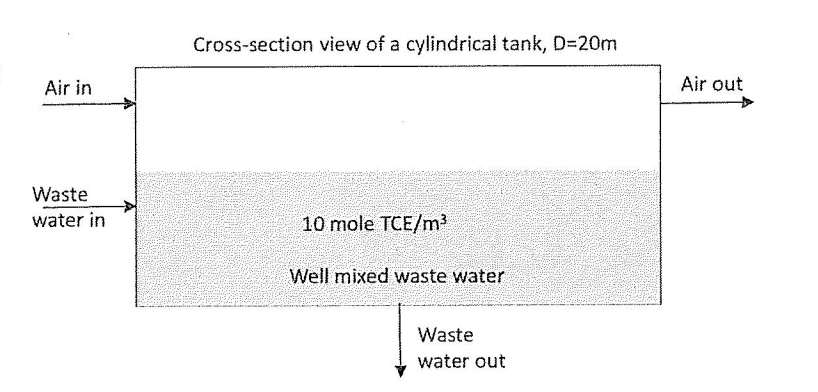




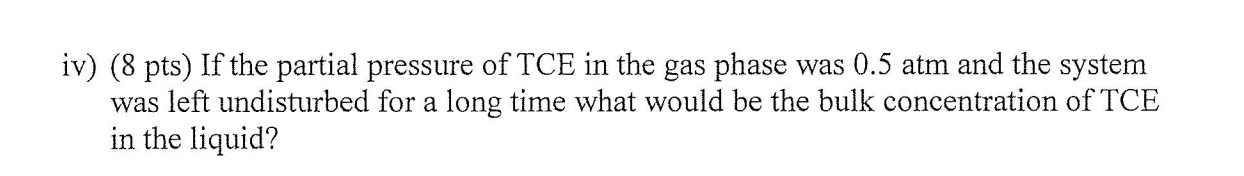



Problem 2. The schematic below shows a waste water treatment process associated with a clarifier. The goal of this waste water treatment process is to decrease the initial concentration of TCE content (initial concentration i $50moleTCE/m3 ) to 10moleTCE/m3 using a stream of fresh air. The clarifier operates an 1.0atm and a constant temperature of 20C. In an independent pilot plant study of TCE, the liquid film mass transfer coefficient for the clarifier was kx=200mole/m2sec, whereas the gas film mass transfer coefficient for the clarifier was ky=0.1mole/m2sec. Equilibrium data for the air-TCE-water system at 20C is represented by Henry's Law with an equilibrium proportionality constant of H=9.9atmL/mol. A. (2 pts) What is the direction of the flux of TCE? Would this be a liquid stripping process or a gas absorption process? B. If the system is stationary (no flow of air or liquid). In this case: i) (2 pts) Would this system reach steady state or equilibrium? Continuing with the stationary system (no flow of air or liquid). In this case: ii) (8 pts) If the system was left undisturbed for a long time and the bulk concentration of TCE in the liquid was kept at 10moleTCE/m3 what would be the partial pressure of TCE in the gas phase? iii) (5 pts) What would be the total concentration of the liquid phase assuming it was only water and TCE? iv) ( 8 pts) If the partial pressure of TCE in the gas phase was 0.5atm and the system was left undisturbed for a long time what would be the bulk concentration of TCE in the liquid? v) (8 pts) Would partial pressure of 0.5atm TCE be a good operations conditions to reach the goal of less than 10 mole TCE/m3 in the effluent? C. (20 pts) Estimate KL or Kx for this system, would it be gas or liquid phase resistant? (35 pts) How much water with less than 10mole/m3TCE in the effluent stream could you produce in the units of m3/s if the partial pressure of TCE in the gasphase is 0.04atm and the inlet concentration is 50mol/m3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


