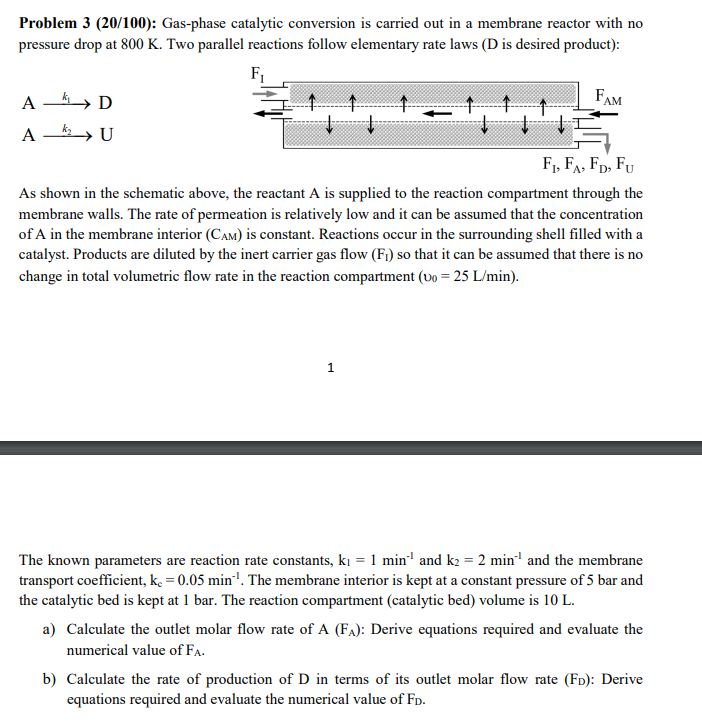
Question: Problem 3 ( 2 0 / 1 0 0 ) : Gas - phase catalytic conversion is carried out in a membrane reactor with no
Problem : Gasphase catalytic conversion is carried out in a membrane reactor with no
pressure drop at Two parallel reactions follow elementary rate laws D is desired product:
As shown in the schematic above, the reactant is supplied to the reaction compartment through the
membrane walls. The rate of permeation is relatively low and it can be assumed that the concentration
of in the membrane interior is constant. Reactions occur in the surrounding shell filled with a
catalyst. Products are diluted by the inert carrier gas flow so that it can be assumed that there is no
change in total volumetric flow rate in the reaction compartment
The known parameters are reaction rate constants, and and the membrane
transport coefficient, The membrane interior is kept at a constant pressure of bar and
the catalytic bed is kept at bar. The reaction compartment catalytic bed volume is
a Calculate the outlet molar flow rate of : Derive equations required and evaluate the
numerical value of
b Calculate the rate of production of in terms of its outlet molar flow rate : Derive
equations required and evaluate the numerical value of
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


