Question: Problem 3 (20 points) - Ideal Gas in a Balloon A balloon is filled with 5mg of helium gas to an initial volume (V1=1.7106m3) and
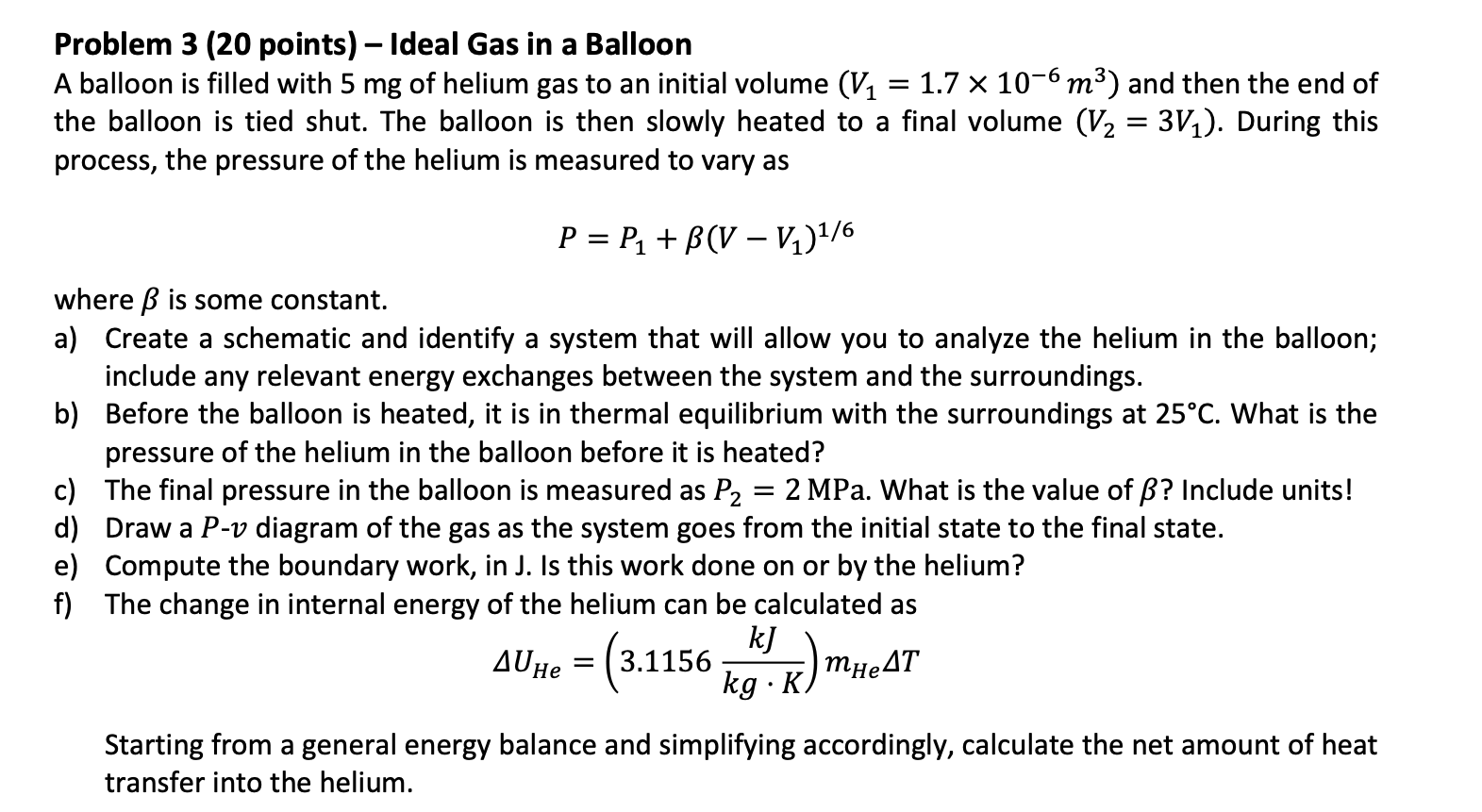
Problem 3 (20 points) - Ideal Gas in a Balloon A balloon is filled with 5mg of helium gas to an initial volume (V1=1.7106m3) and then the end of the balloon is tied shut. The balloon is then slowly heated to a final volume (V2=3V1). During this process, the pressure of the helium is measured to vary as P=P1+(VV1)1/6 where is some constant. a) Create a schematic and identify a system that will allow you to analyze the helium in the balloon; include any relevant energy exchanges between the system and the surroundings. b) Before the balloon is heated, it is in thermal equilibrium with the surroundings at 25C. What is the pressure of the helium in the balloon before it is heated? c) The final pressure in the balloon is measured as P2=2MPa. What is the value of ? Include units! d) Draw a Pv diagram of the gas as the system goes from the initial state to the final state. e) Compute the boundary work, in J. Is this work done on or by the helium? f) The change in internal energy of the helium can be calculated as UHe=(3.1156kgKkJ)mHeT Starting from a general energy balance and simplifying accordingly, calculate the net amount of heat transfer into the helium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


