Question: Problem 3 (33%). The internal energy of a binary mixture of species A and B is given by: Umx = 750 XA + 380 X
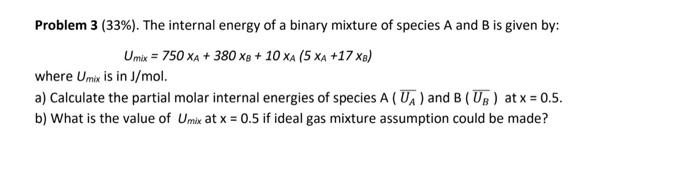
Problem 3 (33%). The internal energy of a binary mixture of species A and B is given by: Umx = 750 XA + 380 X + 10 XA (5 XA +17 xe) where Umix is in J/mol. a) Calculate the partial molar internal energies of species A (UA) and B (Up) at x = 0.5. b) What is the value of Umik at x = 0.5 if ideal gas mixture assumption could be made
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


