Question: Q1(10pts).A 40W heat source is applied to a gas sample for 25s, during which time the gas expands and does 750 J of work on
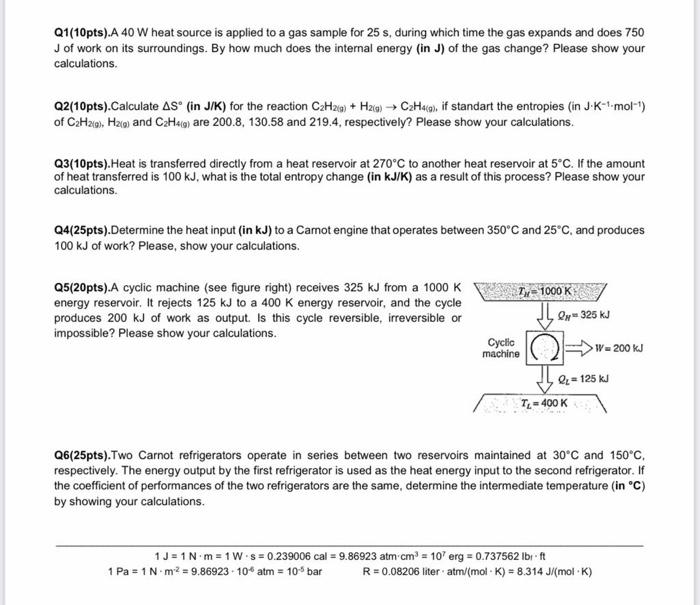
Q1(10pts).A 40W heat source is applied to a gas sample for 25s, during which time the gas expands and does 750 J of work on its surroundings. By how much does the internal energy (in J ) of the gas change? Please show your calculations. of C2H2(9),H2(g) and C2H4(g) are 200.8,130.58 and 219.4, respectively? Please show your calculations. Q3(10pts). Heat is transferred directly from a heat reservoir at 270C to another heat reservoir at 5C. If the amount of heat transferred is 100kJ, what is the total entropy change (in kJ/K ) as a result of this process? Please show your calculations. Q4(25pts).Determine the heat input (in kJ ) to a Camot engine that operates between 350C and 25C, and produces 100kJ of work? Please, show your calculations. Q5(20pts).A cyclic machine (see figure right) receives 325kJ from a 1000K energy reservoir. It rejects 125kJ to a 400K energy reservoir, and the cycle produces 200kJ of work as output. Is this cycle reversible, irreversible or impossible? Please show your calculations. Q6(25pts).Two Carnot refrigerators operate in series between two reservoirs maintained at 30C and 150C, respectively. The energy output by the first refrigerator is used as the heat energy input to the second refrigerator. If the coefficient of performances of the two refrigerators are the same, determine the intermediate temperature (in C ) by showing your calculations. 1J=1Nm=1Ws=0.239006cal=9.86923atmcm3=107erg=0.737562lbft1Pa=1Nm2=9.86923106atm=105barR=0.08206literatm/(molK)=8.314J/(molK) Q1(20pts).In an isothermal reversible process, 2.33 moles of an ideal gas is compressed to one-fifth of its original volume at 285K. What quantity of heat is added to, or removed from, the system during this process? Please, show your calculations. Q2(20pts).An inventor claims that a new heat engine develops 2.1kW of work when heat at the rate of 5.2kJ/s is transferred to the engine. The cycle operates between 1500K and 500K. Evaluate this claim, is it possible? Please, show your calculations. Q3(30pts). Two Carnot refrigerators operate in series between two reservoirs maintained at 20C and 200C, respectively. The energy output by the first refrigerator is used as the heat energy input to the second refrigerator. If the coefficient of performances of the two refrigerators are the same, determine the intermediate temperature by showing your calculations. Q4(30pts).A heat engine operates in a Carnot cycle between reservoirs having temperatures of 1000K and 400K. It is found to discharge 20J of heat per cycle to the cold reservoir. What is the work output per cycle? Please, show your calculations. 1J=1Nm=1Ws=0.239006cal=9.86923atmcm3=107erg=0.737562lbft1Pa=1Nm2=9.86923106atm=105barR=0.08206literatm/(molK)=8.314J/(molK)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


