Question: Problem # 3 A physical solvent is to be used to remove H2S and CO2 from a natural gas stream that contains 10% H2S,5%CO2,80%C1,4.5%C2, and
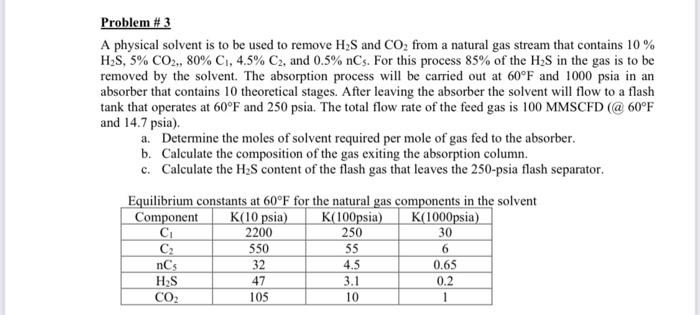
Problem \# 3 A physical solvent is to be used to remove H2S and CO2 from a natural gas stream that contains 10% H2S,5%CO2,80%C1,4.5%C2, and 0.5%nC5. For this process 85% of the H2S in the gas is to be removed by the solvent. The absorption process will be carried out at 60F and 1000psia in an absorber that contains 10 theoretical stages. After leaving the absorber the solvent will flow to a flash tank that operates at 60F and 250 psia. The total flow rate of the feed gas is 100 MMSCFD (@60F and 14.7psia). a. Determine the moles of solvent required per mole of gas fed to the absorber. b. Calculate the composition of the gas exiting the absorption column. c. Calculate the H2S content of the flash gas that leaves the 250 -psia flash separator
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


