Question: Problem 3. An exothermal second-order reaction in a gas phase: A+B (-4) kc4CB k-5*10'exp(-10800/T) m3/(kmol h) takes place in an adiabatic BR with a changeable
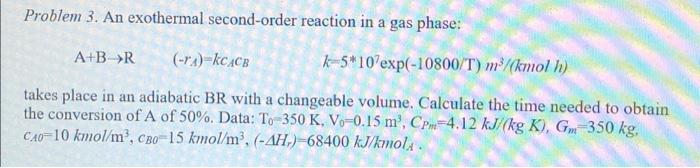
Problem 3. An exothermal second-order reaction in a gas phase: A+B (-4) kc4CB k-5*10'exp(-10800/T) m3/(kmol h) takes place in an adiabatic BR with a changeable volume. Calculate the time needed to obtain the conversion of A of 50%. Data: To-350 K, V-0.15 m, CP=4.12 kJ/(kg K), Gm=350 kg, CA0=10 kmol/m, CB0=15 kmol/m, (-AH.) -68400 kJ/kmolA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


