Question: Problem 3 - Application of first law to closed systems ( 2 7 . 5 pts ) Dr . Bach's bicycle tire has a volume
Problem Application of first law to closed systems pts
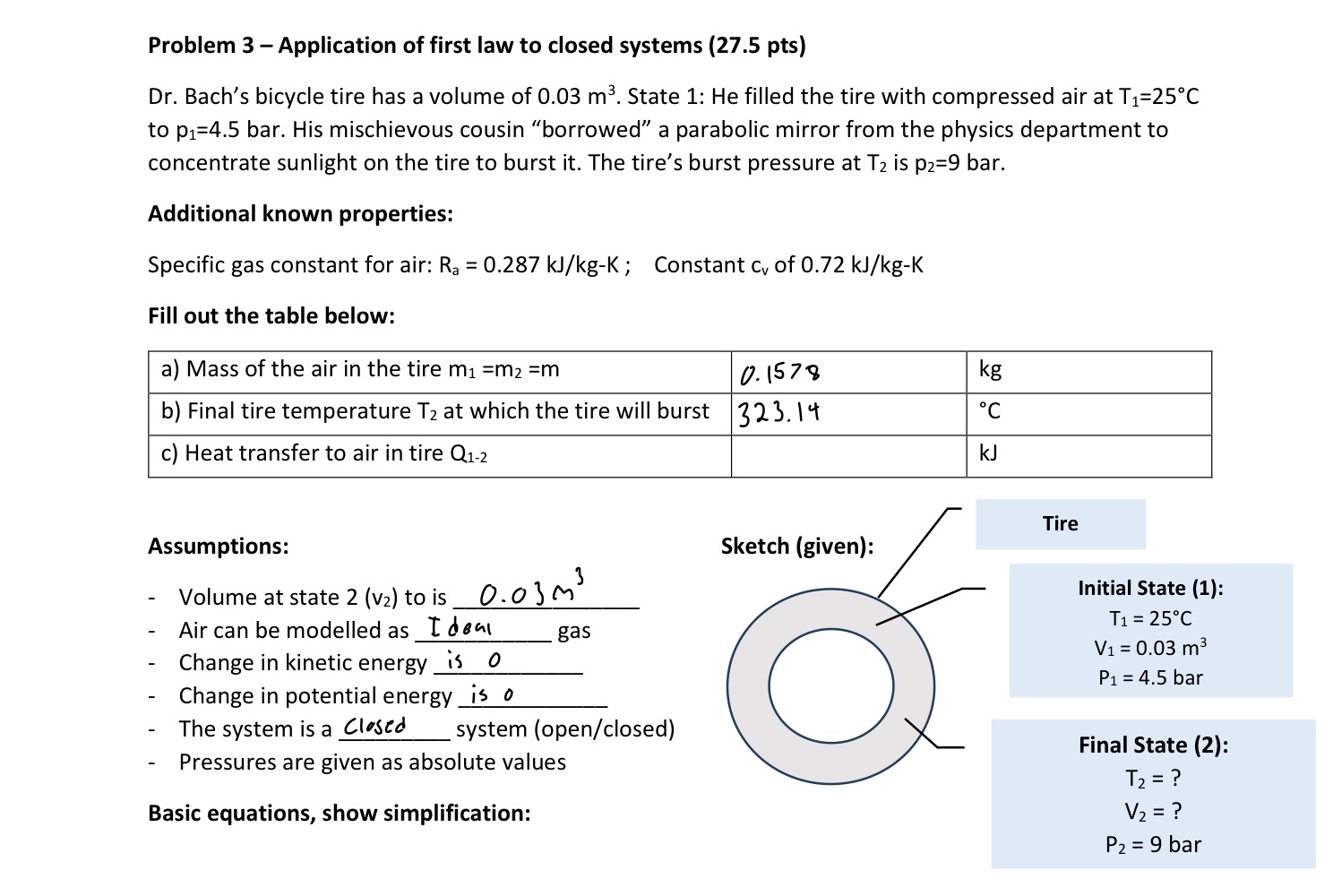
Dr Bach's bicycle tire has a volume of State : He filled the tire with compressed air at to bar. His mischievous cousin "borrowed" a parabolic mirror from the physics department to concentrate sunlight on the tire to burst it The tire's burst pressure at is bar.
Additional known properties:
Specific gas constant for air: ; Constant of
Fill out the table below:
tablea Mass of the air in the tire kgb Final tire temperature at which the tire will burst,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


