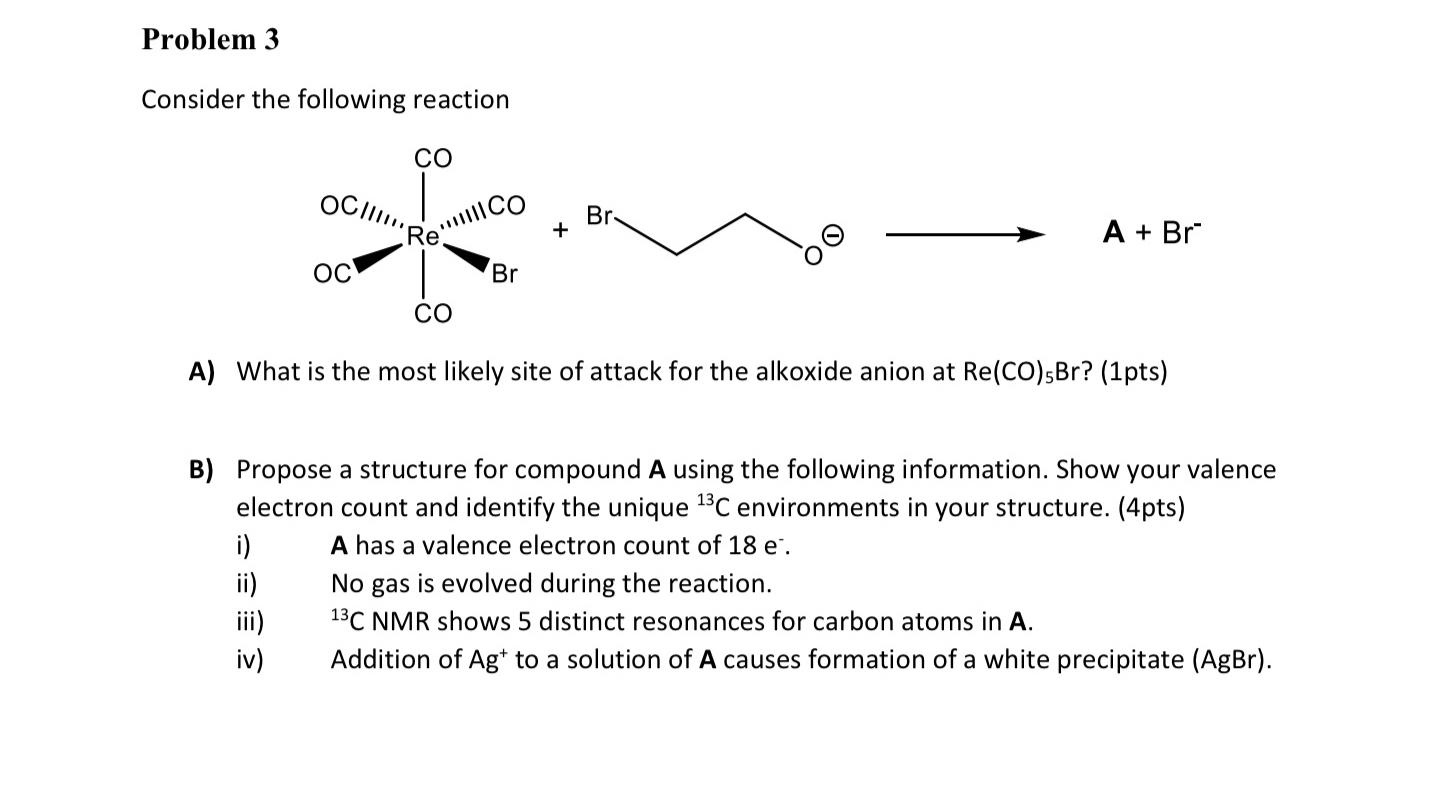
Question: Problem 3 Consider the following reaction A) What is the most likely site of attack for the alkoxide anion at Re(CO)_(5)Br ? (1pts) B) Propose
Problem 3\ Consider the following reaction\ A) What is the most likely site of attack for the alkoxide anion at
Re(CO)_(5)Br? (1pts)\ B) Propose a structure for compound A using the following information. Show your valence electron count and identify the unique
^(13)Cenvironments in your structure. (4pts)\ i) A has a valence electron count of
18e^(-).\ ii) No gas is evolved during the reaction.\ iii)
,^(13)CNMR shows 5 distinct resonances for carbon atoms in
A.\ iv) Addition of
Ag^(+)to a solution of
Acauses formation of a white precipitate
(AgBr).
Consider the following reaction A) What is the most likely site of attack for the alkoxide anion at Re(CO)5Br ? (1pts) B) Propose a structure for compound A using the following information. Show your valence electron count and identify the unique 13C environments in your structure. (4pts) i) A has a valence electron count of 18e : ii) No gas is evolved during the reaction. iii) 13C NMR shows 5 distinct resonances for carbon atoms in A. iv) Addition of Ag+to a solution of A causes formation of a white precipitate ( AgBr)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


