Question: Problem - 3 For an ideal mixture the total pressure can be related to the vapor pressure of the components by P = ? ?
Problem
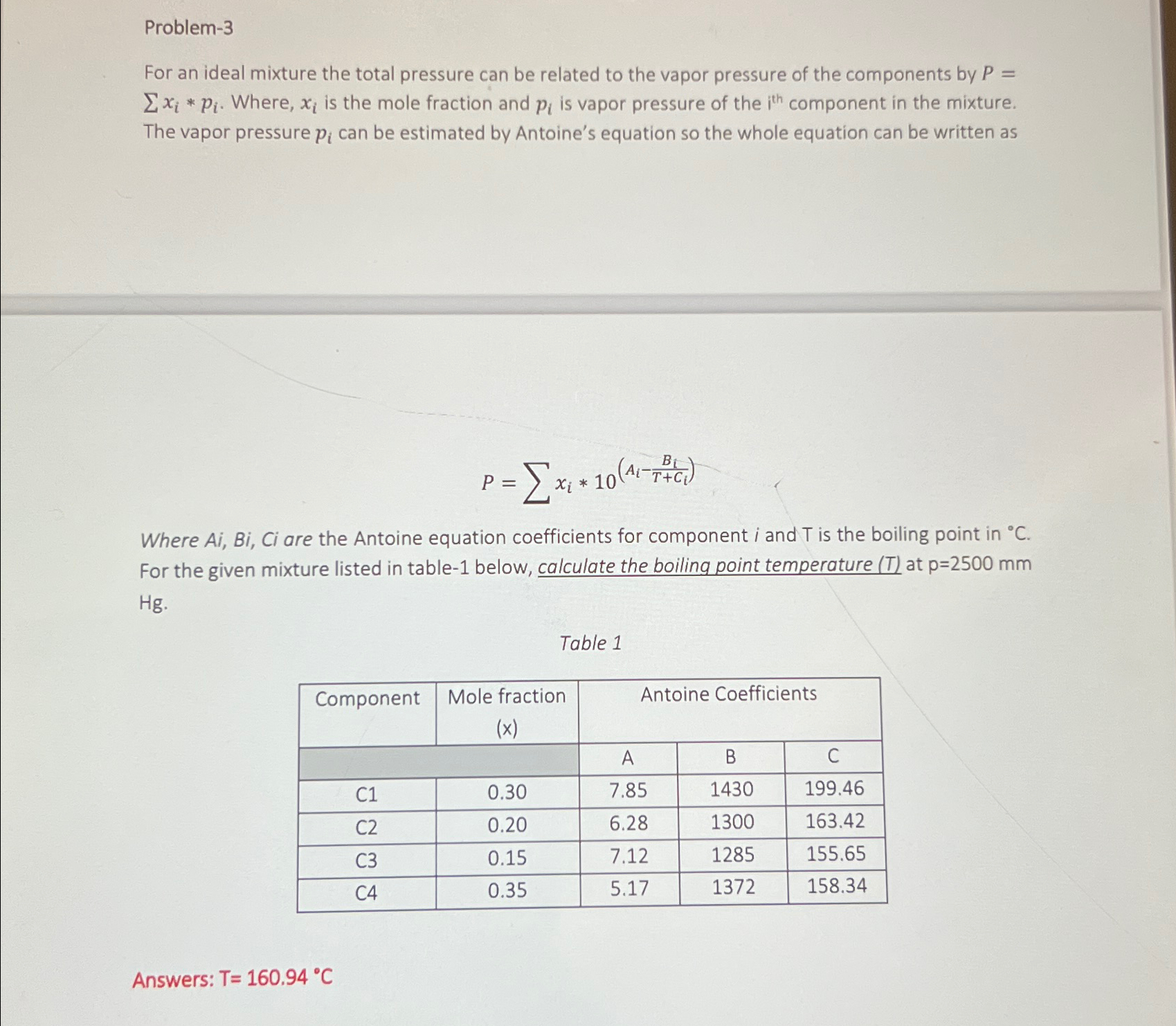
For an ideal mixture the total pressure can be related to the vapor pressure of the components by Where, is the mole fraction and is vapor pressure of the component in the mixture. The vapor pressure can be estimated by Antoine's equation so the whole equation can be written as
Where Ai Bi Ci are the Antoine equation coefficients for component i and is the boiling point in For the given mixture listed in table below, calculate the boiling point temperature at
Table
tableComponenttableMole fraction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


