Question: Problem 3: The following represents experimental data collected by measuring the disappearance of a compound (concentration in mg/L ) with time. Is this a zero-order,
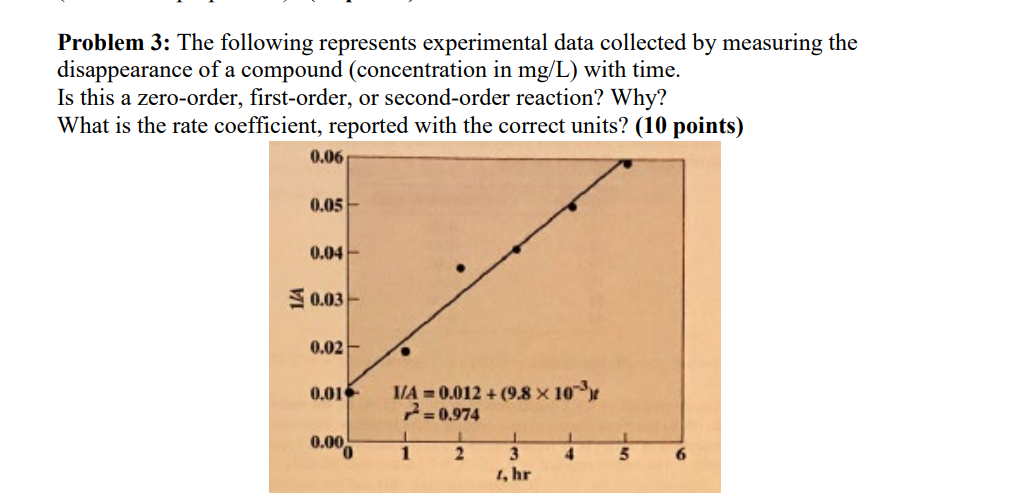
Problem 3: The following represents experimental data collected by measuring the disappearance of a compound (concentration in mg/L ) with time. Is this a zero-order, first-order, or second-order reaction? Why? What is the rate coefficient, reported with the correct units? (10 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


