Question: Problem 3: The semi-empirical mass formula In nuclear physics, the semi-empirical mass formula is a formula for calculating the approximate nuclear binding energy B of
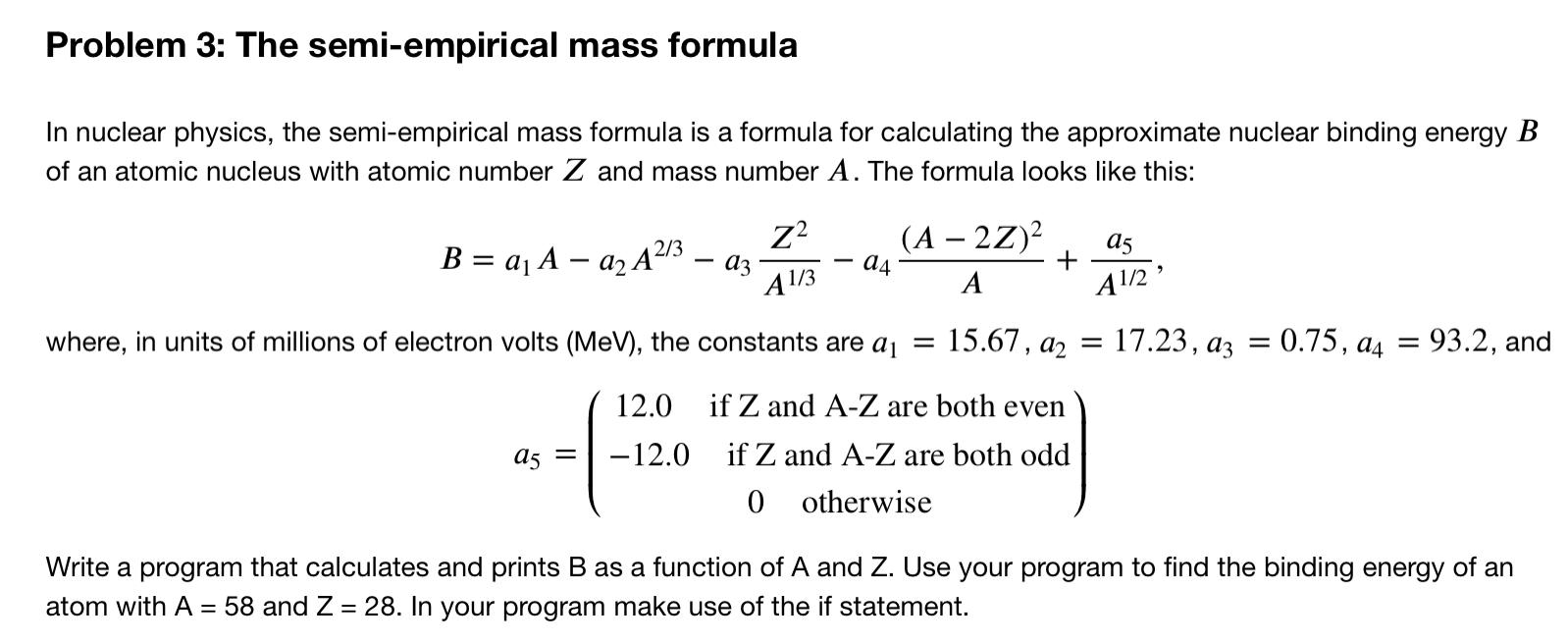
Problem 3: The semi-empirical mass formula In nuclear physics, the semi-empirical mass formula is a formula for calculating the approximate nuclear binding energy B of an atomic nucleus with atomic number Z and mass number A. The formula looks like this: Z? 05 B = ajA az A23 a3 (A 22) - 04 A A1/3 A1/2 ' where, in units of millions of electron volts (MeV), the constants are aj = 15.67, a2 = 17.23, az = 0.75, 04 = = 93.2, and 12.0 A5 = -12.0 if Z and A-Z are both even if Z and A-Z are both od 0 otherwise Write a program that calculates and prints B as a function of A and Z. Use your program to find the binding energy of an atom with A = 58 and Z = 28. In your program make use of the if statement
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


