Question: Problem 3 Through a simulation you obtain a potential energy surface for the ammonia umbrella inversion reaction. You obtain a polynomial t to the potential
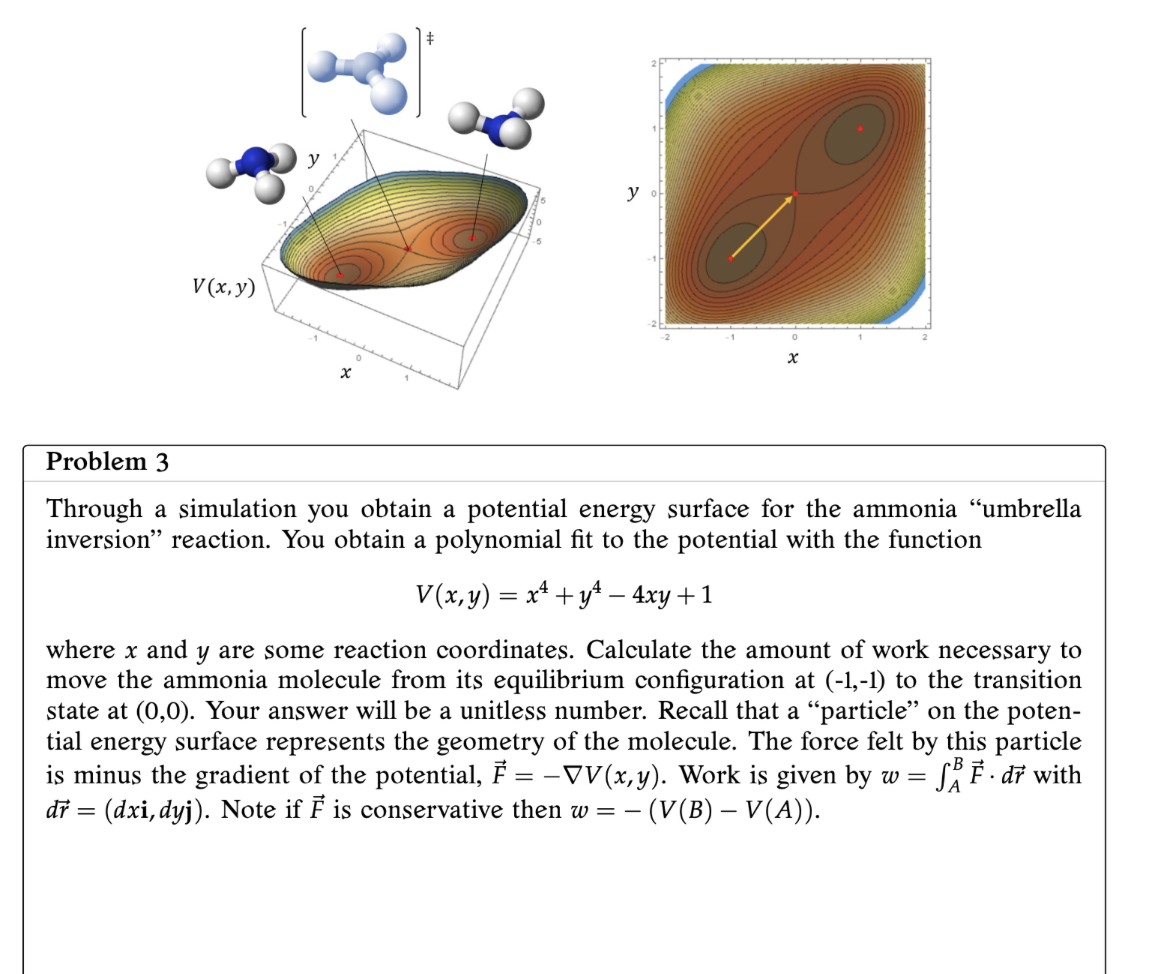
Problem 3 Through a simulation you obtain a potential energy surface for the ammonia \"umbrella inversion\" reaction. You obtain a polynomial t to the potential with the function V(x,y) = x4+y44xy+1 where x and y are some reaction coordinates. Calculate the amount of work necessary to move the ammonia molecule from its equilibrium conguration at (1,1) to the transition state at (0,0). Your answer will be a unitless number. Recall that a \"particle\" on the poten- tial energy surface represents the geometry of the molecule. The force felt by this particle is minus the gradient of the potential, 1'5 = VV(x,y). Work is given by w = f: P. - d? with d? = (dxi,dyj). Note if 15 is conservative then to = (V(B) V(A))
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


