Question: Problem 3-Clausius Inequality (8 pts) The table below shows different processes occurring in a power cycle operating steadily between a hot and cold reservoirs
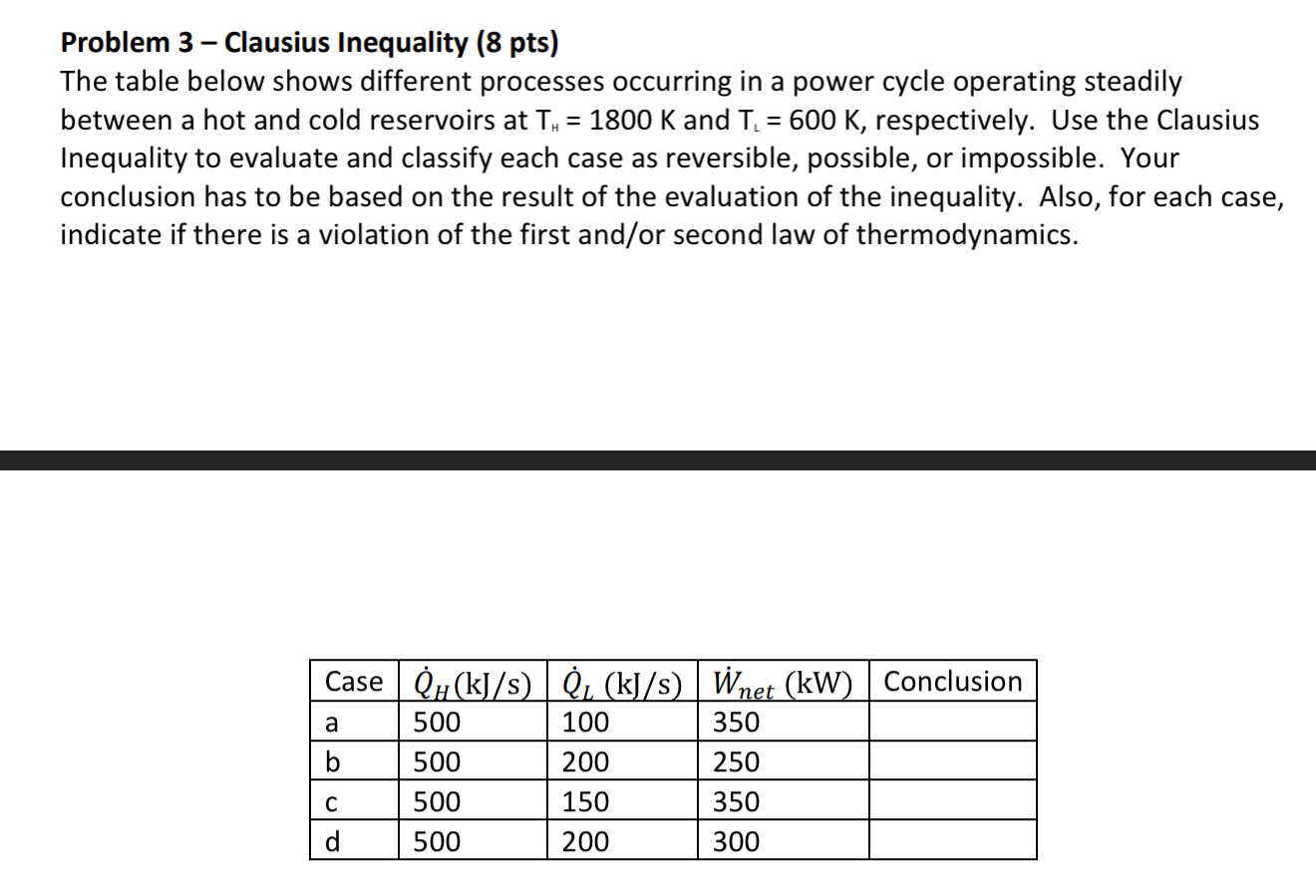
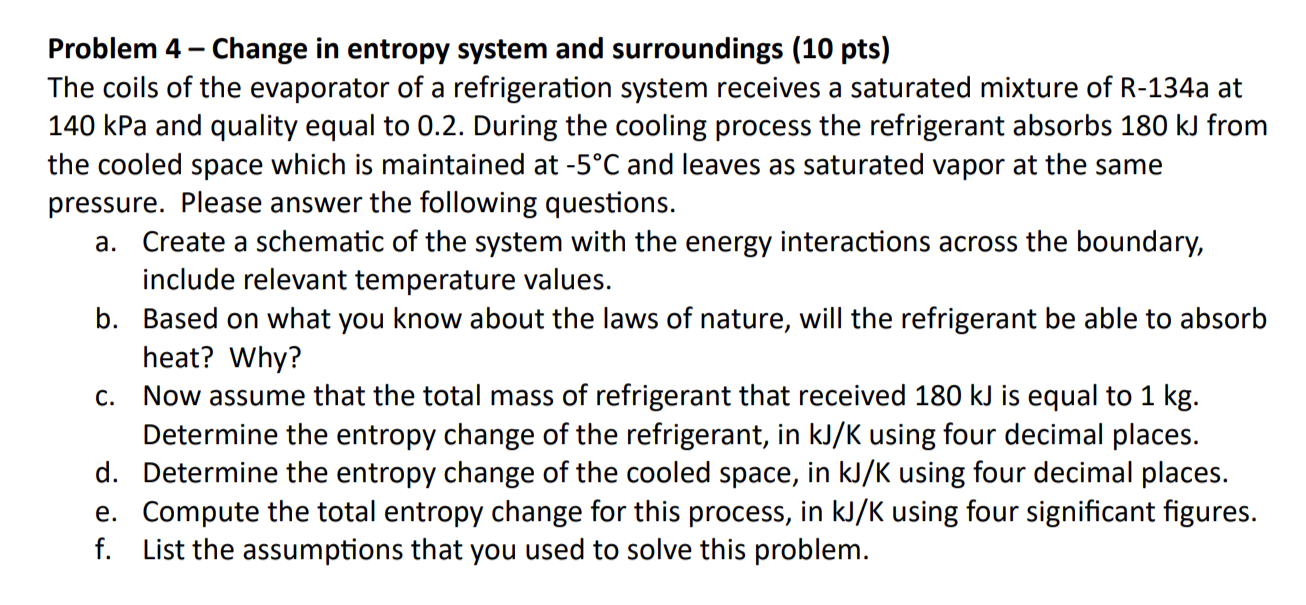


Problem 3-Clausius Inequality (8 pts) The table below shows different processes occurring in a power cycle operating steadily between a hot and cold reservoirs at T = 1800 K and T = 600 K, respectively. Use the Clausius Inequality to evaluate and classify each case as reversible, possible, or impossible. Your conclusion has to be based on the result of the evaluation of the inequality. Also, for each case, indicate if there is a violation of the first and/or second law of thermodynamics. Case QH (kJ/s) QL (kJ/s) Wnet (kW) Conclusion a 500 100 350 b 500 200 250 C 500 150 350 d 500 200 300 Problem 4 - Change in entropy system and surroundings (10 pts) The coils of the evaporator of a refrigeration system receives a saturated mixture of R-134a at 140 kPa and quality equal to 0.2. During the cooling process the refrigerant absorbs 180 kJ from the cooled space which is maintained at -5C and leaves as saturated vapor at the same pressure. Please answer the following questions. a. Create a schematic of the system with the energy interactions across the boundary, include relevant temperature values. b. Based on what you know about the laws of nature, will the refrigerant be able to absorb heat? Why? C. Now assume that the total mass of refrigerant that received 180 kJ is equal to 1 kg. Determine the entropy change of the refrigerant, in kJ/K using four decimal places. d. Determine the entropy change of the cooled space, in kJ/K using four decimal places. e. Compute the total entropy change for this process, in kJ/K using four significant figures. f. List the assumptions that you used to solve this problem. Problem 5 - Change in entropy for a pure substance (10 pts) A frictionless piston-cylinder assembly contains 0.005 m water at 150 kPa and 225C. Next, the water is cooled at constant pressure until its quality becomes equal to 0.70. Please answer the following. a. Represent the process for the water on a T-s diagram. In your T-s diagram include values for P, T and s. b. Determine the entropy change of the water during this process, in kJ/K using three significant figures. c. Determine the amount of heat removed from the water, in kJ. Problem 6 - Isentropic process (5 pts) R-134a is initially at 600 kPa and 25C. It undergoes a process during which the entropy is kept constant. The final pressure is 200 kPa. What are the temperature and specific enthalpy at the final state?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


