Question: Problem 4 A piston-cylinder assembly has the following components: - A piston of negligible mass and a cross-sectional area of 0.1m2. - A set of
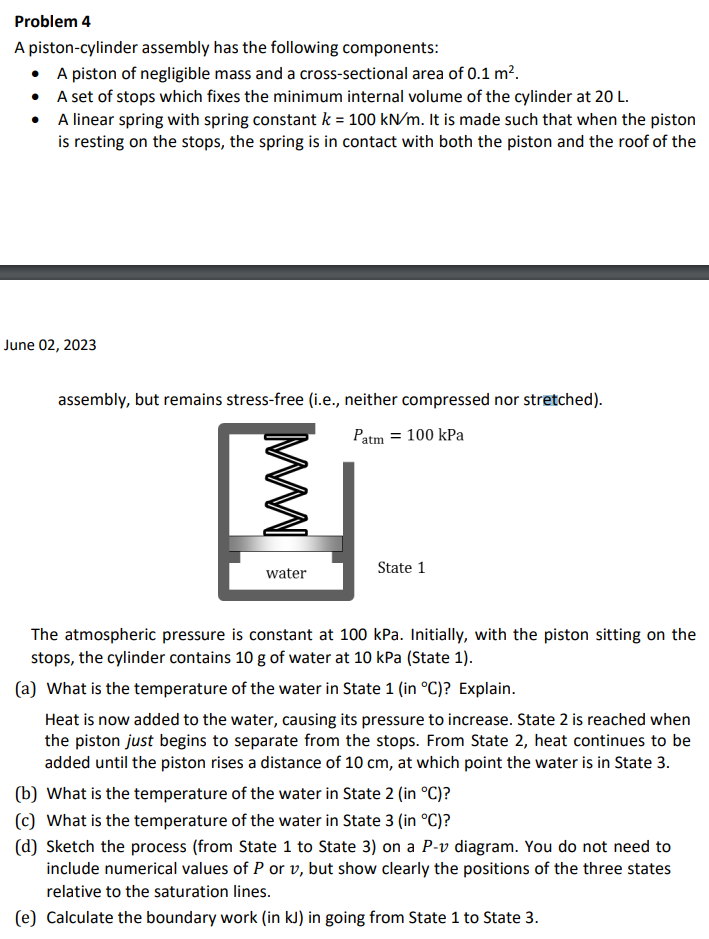
Problem 4 A piston-cylinder assembly has the following components: - A piston of negligible mass and a cross-sectional area of 0.1m2. - A set of stops which fixes the minimum internal volume of the cylinder at 20L. - A linear spring with spring constant k=100kN/m. It is made such that when the piston is resting on the stops, the spring is in contact with both the piston and the roof of the June 02, 2023 assembly, but remains stress-free (i.e., neither compressed nor stretched). The atmospheric pressure is constant at 100kPa. Initially, with the piston sitting on the stops, the cylinder contains 10g of water at 10kPa (State 1). (a) What is the temperature of the water in State 1 (in C) ? Explain. Heat is now added to the water, causing its pressure to increase. State 2 is reached when the piston just begins to separate from the stops. From State 2, heat continues to be added until the piston rises a distance of 10cm, at which point the water is in State 3. (b) What is the temperature of the water in State 2 (in C) ? (c) What is the temperature of the water in State 3 (in C) ? (d) Sketch the process (from State 1 to State 3) on a Pv diagram. You do not need to include numerical values of P or v, but show clearly the positions of the three states relative to the saturation lines. (e) Calculate the boundary work (in kJ) in going from State 1 to State 3. Problem 4 A piston-cylinder assembly has the following components: - A piston of negligible mass and a cross-sectional area of 0.1m2. - A set of stops which fixes the minimum internal volume of the cylinder at 20L. - A linear spring with spring constant k=100kN/m. It is made such that when the piston is resting on the stops, the spring is in contact with both the piston and the roof of the June 02, 2023 assembly, but remains stress-free (i.e., neither compressed nor stretched). The atmospheric pressure is constant at 100kPa. Initially, with the piston sitting on the stops, the cylinder contains 10g of water at 10kPa (State 1). (a) What is the temperature of the water in State 1 (in C) ? Explain. Heat is now added to the water, causing its pressure to increase. State 2 is reached when the piston just begins to separate from the stops. From State 2, heat continues to be added until the piston rises a distance of 10cm, at which point the water is in State 3. (b) What is the temperature of the water in State 2 (in C) ? (c) What is the temperature of the water in State 3 (in C) ? (d) Sketch the process (from State 1 to State 3) on a Pv diagram. You do not need to include numerical values of P or v, but show clearly the positions of the three states relative to the saturation lines. (e) Calculate the boundary work (in kJ) in going from State 1 to State 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


