Question: Problem 4. Apparent deviations from Beer's Law arise when an analyte dissociates, associates, or reacts with a solvent to produce a product having a different
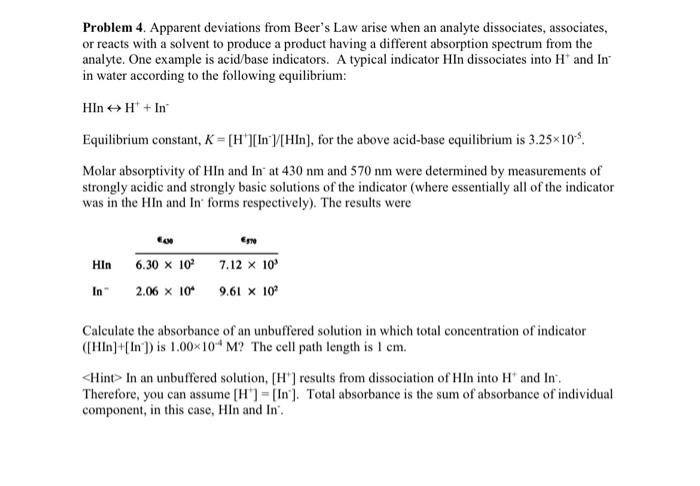
Problem 4. Apparent deviations from Beer's Law arise when an analyte dissociates, associates, or reacts with a solvent to produce a product having a different absorption spectrum from the analyte. One example is acid/base indicators. A typical indicator HIn dissociates into H+and In in water according to the following equilibrium: HInH++In Equilibrium constant, K=[H+][In]/[HIn], for the above acid-base equilibrium is 3.25105. Molar absorptivity of HIn and In at 430nm and 570nm were determined by measurements of strongly acidic and strongly basic solutions of the indicator (where essentially all of the indicator was in the HIn and In' forms respectively). The results were Calculate the absorbance of an unbuffered solution in which total concentration of indicator ([Hln]+[ln])is 1.00104M ? The cell path length is 1cm.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


